Preventive Conservation: Agents of Decay
D. Gilroy and I. M. Godfrey
Introduction
The simplest and most inexpensive way to look after an object or collection is to prevent it suffering deterioration in the first place. Whether an object is in a home or in a museum, the same causes of deterioration apply. A metal artefact in a garden shed will suffer more damage than it would if stored in a box inside a house. Similarly a painting on display in a home or gallery may deteriorate more if placed into a less favourable museum storage environment. There is no point in spending time and materials to treat an object if it is returned to the same conditions which led to the original deterioration.
Information presented in this section concentrates on the environmental factors which cause deterioration in materials. Different climatic zones have their own distinct problems. High humidity in the tropics and salt-laden air in coastal regions are typical examples which should receive extra consideration. Other obvious dangers to collections include fire, flooding and theft. Although these factors must be considered by those responsible for a collection they are not covered in detail in this book.
It is important to know from what materials an object is made and their susceptibility to the various causes of decay. This information is useful in determining where and how it should be stored or displayed.
Thorough preventive conservation practices should be established and regular inspections are essential if the best conditions for a collection are to be maintained. It is important, for example, not to take food or drink into display or storage areas. Spillages have the potential to not only damage objects but also to attract potentially damaging pests. Apart from the obvious health issues, smoking is not recommended in these areas because of the risk of damage from either fire or smoke.
Another important issue is the level of access to artefacts. Materials used daily for educational purposes should be copies or non-essential artefacts because constant handling will cause deterioration through either breakage or general wear and tear.
To reduce the risk of cross contamination in storage and display areas, any new material coming into a collection should be isolated initially, inspected and if necessary, cleaned. Treatment of pests should be arranged if infestations are found.
The most significant environmental factors which contribute to the degradation of objects are listed below:
- light;
- temperature;
- relative humidity (RH);
- pollutants; and
- biological pests.
Many of these factors are interrelated and cannot be considered separately. Bright sunlight for example will cause photochemical damage to light-sensitive material, increased temperature and decreased relative humidity.
Each of these agents of deterioration will be discussed in turn in the following sections with guidelines given regarding the types of conditions that will enhance the longevity of materials. As most collections are made up of an assortment of objects and material types no one set of conditions will be suitable for all objects. Where a choice has to be made, conditions should be tailored to minimise damage to the most sensitive of the objects.
Light
Principles
Light is an energy form capable of damaging materials such as organic fibres (textiles and paper), watercolour paintings, dyed materials, coloured leather, botanical specimens and colour photographs.
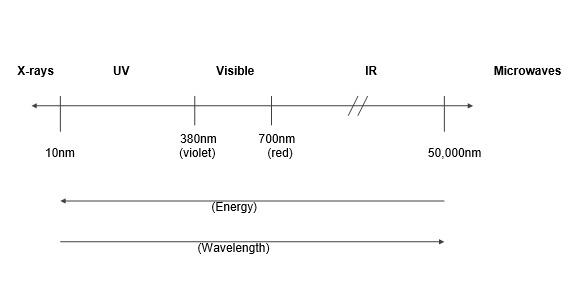
The human eye can detect only those wavelengths of light that make up the visible spectrum (rainbow colours). Unseen components of light such as ultraviolet (UV) and infra-red (IR) light can also severely affect particular material types (Figure 1).
Figure 1: Light region of the electromagnetic spectrum.
Ultraviolet (UV) Radiation
(10-380 nm)
As wavelengths below 300 nanometres (nm) do not penetrate the earth's atmosphere and glass cuts out UV radiation with wavelengths of less than 320 nm, the UV band of concern is 320-380 nm. This shortwave, invisible UV radiation is highly energetic and is most likely to cause photochemical deterioration. There is a high proportion of UV radiation in daylight and a significant amount is emitted by fluorescent tubes.
Visible Light
(380-760 nm)
Visible light affects an object in two important ways - it can lead to its deterioration and it can affect the way an observer perceives an object.
Deterioration is caused not only by high energy light at the blue end of the spectrum but also by heat produced by lower energy red light. Exposure to brighter, more intense light increases the rate at which light-induced damage occurs.
If colour-corrected light is not used for viewing, a colour shift may occur that human eyes cannot perceive. Examples of this include the apparent change in colour when red meat is removed from specially lit butchers’ displays (metamerism) into the ordinary light of their stores and the yellow tinge imparted to objects by a dimmed incandescent globe. Fluorescent tubes on the other hand, are an excellent source of accurate colour-rendering light. Unfortunately this same light is capable of causing significant photochemical damage to susceptible objects.
Infra-red (IR) Radiation
(760 nm upwards)
IR radiation can cause objects to heat up and accelerate chemical deterioration processes. It is also capable of producing changes in relative humidity levels. Under conditions of reduced relative humidity objects can become brittle, while increased relative humidity will increase corrosion of metals. Such changes in relative humidity correspond to the IR source being turned on and off respectively.
Light Measurement
The human eye responds well to green-yellow light but less so to the blue and red ends of the spectrum. Both UV and IR radiation are invisible to the human eye. Light meters and cameras have been designed to measure visible light in the same way that the eye perceives it.
The brightness or intensity of light is measured in units called lumens. One lumen every square metre is equivalent to one lux. When measuring light intensity the meter must face the direction of the light source. To determine the intensity of light impacting on an object, place the meter directly in front of the object, as close to the surface as possible and parallel to the object’s surface.
UV radiation may be measured either as a proportion of visible light, with units of microwatts per lumen (µW/lumen) or more usefully as a direct measure of actual UV exposure, with units of microwatts per square metre (µW/m2).
IR radiation is not usually measured, with its effect most easily detected by monitoring associated temperature changes.
Advances in technology have led to the development of light and UV monitoring devices that can take either one-off, instantaneous measurements or, by the use of meters with data logging capabilities, can monitor these parameters over extended periods. Data logging instruments allow monitoring of light variations that occur during the day and as the angle of the sun alters from season to season. Use of these instruments to determine light exposure profiles aids in the long-term management of light sensitive objects.
While specialist equipment is available to monitor light and UV levels, the brightness of visible light can also be measured easily using any camera that has a built-in light meter (see Appendix 1).
If, as is often the case, light and UV monitors are not readily available, it is important to be aware of the approximate light and UV radiation levels generated by various light sources. Note that the actual levels of exposure are dependent on the distance between the light source and the exposed object, with increasing distance resulting in reduced light and UV levels.
| Examples of illumination (visible light) levels: | Lux |
|---|---|
| Daylight, fluorescent lights in an office or laboratory | 600-1500 |
| Daylight coming in through a window | 35,000 |
| Direct sunlight | up to 136,000 |
| Examples of UV from various sources: | µW/lumen |
|---|---|
| Fluorescent lights (low UV) | 33 |
| Fluorescent lights (general) | 80-250 |
| Tungsten globe | 60-80 |
| Direct sun | 400 |
| Light overcast sky | 800 |
| Blue sky | 1600 |
If the light intensity (lux) and the UV radiation levels (µW/lumen) are known then the actual UV exposure (µW/m2) can be calculated by multiplying these two values together. For example, a fluorescent tube with a light intensity of 800 lux and a UV output of 100 µW/lumen would be exposing an object to UV levels corresponding to 80,000 µW/m2 (= 800 lumens/m2 x 100 µW/lumen).
Another important aspect of light measurement relates to the measurement of colours in objects. Although this requires the use of specialised equipment (Chroma Meters or colorimeters), it provides an accurate way of quantifying changes that may occur in coloured objects, paintings and prints. In this process, measurements taken at strategic points are repeated after a period of light exposure. Any changes in colour will be detected and can be related to the environmental conditions to which the object was exposed. Chroma Meters range in price and quality and it is possible to use one instrument to measure chromaticity, colour difference, correlated colour temperature and the luminance of light sources.
Light-induced Deterioration
Light has many effects, the most noticeable of which are colour changes induced in objects. For instance the bleaching of ivory, the fading of pigments, dyes and inks and the discolouration of wood, varnishes and lacquers are all due to light exposure (Figure 2).
Different materials react to light in different ways. Some materials fade while others darken. Some types of wood yellow, others bleach and some turn grey when exposed to light. A more subtle effect can be observed in colour photographs or water colours where there may be a colour shift when one of the more light-sensitive dyes is affected by light.
In addition to inducing colour changes in objects, exposure to light is also responsible for weakening fibres in textiles and paper.
Light has the potential to damage organic materials whether they are in pure form or make up part of a composite object. Natural organic materials such as wood, fibres and biological specimens are all vulnerable, as are modern polymeric materials (plastics) and even metal objects which contain an organic component such as paint, lacquer or an inlay. The inlaid ivory handle of a hand gun for instance, would be susceptible to light damage.
For composite objects the most sensitive component must be considered when planning for display and/or storage. The relative sensitivities of different materials may be gauged by comparing the recommended maximum lighting levels for these materials (see below). Expose the most sensitive objects to lower light levels to minimise photochemical damage.
Reciprocity Rule
The same amount of damage will be produced by strong light in a short time as will be done by a weaker light over a longer period. For example, 500 lux exposure for 10 hours will cause an equivalent amount of deterioration to 50 lux exposure for 100 hours.
Monitoring Deterioration
The impacts of lighting conditions can be monitored on site using light dosimeters such as blue wool scales or the recently developed LightCheck®. A blue wool scale is a small card comprising a series of dyes of differing light fastness (Figure 2). Half of the card is covered and it is then placed in the area in which lighting impacts are to be determined. After a fixed exposure period, the blue wool scale is removed and the cover removed from the card. A comparison can then be made between the exposed and unexposed dyes and a clear indication gained as to the likely damage that will occur to light sensitive objects exposed at the test site.
Figure 2: A blue wool test strip where the left side of the strip was covered while the right side was exposed to full sun for 30 days (Copyright Catharine Ellis).
An alternative system, LightCheck®, can be used in a similar fashion. A LightCheck® strip is placed next to an object on display and regularly inspected. The extent of light damage is determined by comparison with a colour scale that can then be related to an equivalent exposure level. LightCheck® strips are available in two formats, one for shorter duration and testing in areas holding very light sensitive objects and the other for areas in which less sensitive objects are exposed. The LightCheck® strips are considered to be more sensitive to light exposure than the blue wool scales.
Recommended Maximum Light and UV Levels
These values are set so that objects can be viewed while minimising the risk of photochemical damage. Note that the UV levels recommended below correspond to 30 µW/lumen for highly sensitive objects and 75 µW/lumen for sensitive and insensitive objects.
Highly Sensitive Objects - 50 lux and 1500 µW/m2
Textiles, costumes, watercolours, tapestries, prints and drawings, manuscripts, miniatures, distemper in frescoes, wallpapers, gouache, dyed leather, most natural exhibits, botanical specimens, fur and feathers.
Sensitive Objects - 200 lux and 15,000 µW/m2
Oil, tempera and acrylic paintings, undyed leather, horn, bone, ivory, most wood, oriental lacquer and other painted or coloured objects.
Light Insensitive Objects - 300 lux and 22,500 µW/m2
Stone, metal, glass, ceramic, jewellery, enamel and wooden objects that have largely been used outdoors or have otherwise lost their natural colouring.
Recommended Overall Exposure Levels
When developing a lighting strategy it is essential to consider overall exposure levels and not just maximum light and UV levels. Overall exposure levels can be determined by multiplying the actual light exposure levels by the number of hours for which objects are exposed. For example, an object exposed to an average 50 lux for 8 hours a day, 300 days of the year would have an overall exposure of 50 x 8 x 300 lux hours (= 120,000 lux hours or 120 klux hours). For highly light sensitive objects, Thomson (1986) proposes overall exposures of 200 klux hours, for sensitive objects 650 klux hours and no overall limit for light insensitive objects. For these latter objects overall light levels will be largely determined by the environmental requirements of nearby objects.
Determining overall exposure levels is not easy however and may require extensive data logging. If daylight impinges on display spaces, consultation with local meteorological offices or educational institutions may be necessary to determine daylight availability. Daylight availability varies with the changing seasons due to the duration of available light and the angle of the sun.
Controlling Light Levels
Of all the agents that contribute to the deterioration of cultural materials, light is probably the easiest to control. The problem is achieving the right balance between minimising photochemical damage by limiting light levels and still maintaining appropriate colour rendering of objects and allowing objects to be viewed without reducing surface details. Loss of object detail can be a significant problem for older visitors to museums.
Light levels may be controlled by using some or all of the following strategies:
- exclude all daylight;
- place the most light-sensitive objects furthest from light sources;
- use low UV light sources;
- use UV filters over light sources or in display cabinets;
- alternate sensitive materials between display and low-light storage;
- use partitions to create ‘shaded’ areas;
- use copies for display; and
- use automatic light switching.
Ideally, all daylight should be excluded from storage and display areas. This has three beneficial effects. It reduces overall light levels significantly, it allows the intensity of interior light and the amount of UV exposure to be controlled and also gives greater opportunities for creative lighting effects to be introduced.
Light control may be achieved by using appropriate artificial lighting sources such as low UV fluorescent lights and arrangements such as reflected rather than direct lighting. Whatever the light sources and arrangements, the end result must be conditions that meet the required specifications for the illuminated objects and allow the objects to be viewed satisfactorily.
Options to eliminate daylight include the use of blinds, shutters, curtains and even paint. While UV-absorbing films and tints can also play a part in reducing overall light and UV levels, the former methods are preferred. It is obvious when curtains, blinds and shutters have deteriorated to the point that they are no longer effective in reducing light intensities and need to be replaced, but it is often more difficult to determine when UV absorbing films are no longer performing to their original specifications. Neutral density films can also be applied to windows. These will reduce the overall light transmission and, as long as they are applied to all windows in a particular space, will create the impression that the windows are all clear.
As the intensity of light is reduced with distance from a light source, place the most light-sensitive objects furthest from it.
The same result may be achieved by bouncing light off a reflecting surface to create a diffuse effect. The intensity of the light is reduced because the path length of the light from the source to the object is increased, some of the incident light is absorbed and some is scattered by the reflecting surface. Depending on the nature of the reflecting surface, this is an excellent way of not only reducing light intensity but also of reducing UV levels in the reflected light and creating interesting lighting effects. When daylight is reflected from white walls, usually containing titanium dioxide pigments, approximately 80 % of the UV radiation is absorbed during each reflection.
Options to reduce the impact of UV radiation on objects include the use of low UV fluorescent tubes, installation of UV absorbing covers or sleeves over light sources and the use of UV absorbing perspex or UV absorbing films in display case construction. The use of a diffuser over a fluorescent tube for instance, will usually reduce the UV radiation to acceptable values. This is because most diffusers have UV absorbing chemicals in them to prevent their deterioration when in use.
Light exposure can also be reduced by adopting appropriate management strategies. Material that is particularly light sensitive may be alternated between display and storage. To cut light exposure by half in an historic house for example, only open half of the rooms for six months of the year and the other half for the following six months. Similarly, if a diary or register is displayed open, then rotate the exposed pages.
Another alternative is to use lights that are activated when someone enters the collection area room and which remain on for a limited period (automatic light switching).
Note that the importance of an object as well as its sensitivity to light damage must be considered carefully before remedial measures are taken. Occasionally, maximum light levels are insufficient to show artefact details adequately. This problem may arise when general light levels in the vicinity are too high, making the artefact at say 50 or 200 lux, relatively dark. Decreasing general light levels in the vicinity of the artefact will assist in overcoming this problem.
If the light on an artefact must be increased above recommended levels then reduce the display time proportionately. For example if the recommended light level is 50 lux and the actual level is 200 lux then put the object on display for only three months per year and keep it in dark storage for the other nine months.
If appropriate light levels cannot be established then make copies of particularly sensitive or significant photographs, prints and similar objects. The copy may then be put on display and the original safely stored.
Artificial Light Sources
There are many factors to be taken into account when considering the use of artificial light sources. These include:
- the light source itself;
- how the light gets from the source to the object; and
- maintenance and control systems.
When choosing a light source, factors to be considered include the ability of the source to render colours accurately, its colour temperature and its UV output. The standard measure for colour rendering is the colour rendering index (CRI) with daylight being given a CRI of 100. At present, tungsten halogen lights, with a CRI of 99 and some (not all) fluorescent lights with CRIs in the 90s are the first choices for museum lighting systems.
Note that light sources should not be in enclosed spaces with objects. Heat generated by the ballast of fluorescent lights, by tungsten lights themselves or by the motors of fibre optic systems can build up to damaging levels.
Light emitting diode (LED) technology is developing rapidly and it is envisaged that these light sources will become much more widely used in museum displays and in general spaces. LEDs can be combined to give a full colour spectrum, are UV free, use less energy than traditional halogen sources and because of their greater longevity, reduce on-going maintenance and costs.
While a colour temperature of 3000 K has been recommended for museum light sources, the human visual system responds to illumination in a discriminatory fashion. It has been demonstrated for instance, that the colour temperature of light sources preferred by observers varies according to the dominant colours in the object being viewed. Because of this it is strongly recommended that the same type of light source be used in areas that can be viewed simultaneously.
The most usual way of getting light from the source to the object is via a reflector, with most light sources sitting within a reflector in a light fitting. The type of reflector will affect the shape of the beam and will also control any stray light. Reflectors should be cleaned regularly.
Consult lighting specialists if more sophisticated delivery systems, including those using diffusing or ribbed glass lenses and fibre optics are considered for lighting displays. Glass fibre optic systems have some particular advantages in museum lighting, allowing the distribution of light from one lamp over a greater area, the placement of the lamp at a distance from the lit object and a natural reduction in the UV content of the light along the way. They are not a cure-all however, simply another tool to be considered.
Lighting systems should be designed so that maintenance and control are facilitated with fittings suitable for extended use and for easy lamp changes. Centralised controls and dimming facilities can allow for better overall control over light levels and the transition between differently lit areas. It is worth noting however, that strong dimming of tungsten halogen lights reduces their CRI.
As there are a wide variety of light sources available and lighting technology is continually developing, consultation with manufacturers and suppliers is recommended so that the latest and most appropriate information can be obtained.
Relative Humidity and Temperature
These deterioration factors are considered together because of their close inter-relationship.
Principles
The relative humidity (RH) of the air is an indication of how much water vapour is in the air at a particular temperature compared with how much water vapour the air could actually hold at that temperature. It is expressed as a percentage and can be defined as follows:
| RH = | amount of water in a given amount of air | x | 100 |
| max. amount of water the air can hold at that temperature | 1 |
Air at 100 % relative humidity holds the maximum amount of water possible at that particular temperature and is said to be saturated. Saturated air at 10 °C holds about 10 grams per cubic metre (g/m3) of moisture; at 20 °C about 17 g/m3 and at 30 °C more than 30 g/m3. Put simply, the relative humidity is a measure of the percentage saturation of the air. Therefore air at 50% relative humidity, regardless of temperature, is holding half of its total possible water capacity.
In essence, cold air cannot hold as much water vapour as warm air. In a closed environment such as a display case, there will be a fixed amount of water vapour, referred to as the absolute humidity. If the temperature inside the case falls then the relative humidity will rise. If the temperature rises the relative humidity will fall. Such changes in relative humidity could be caused by many factors including direct sunlight, spotlights and air-conditioning failures.
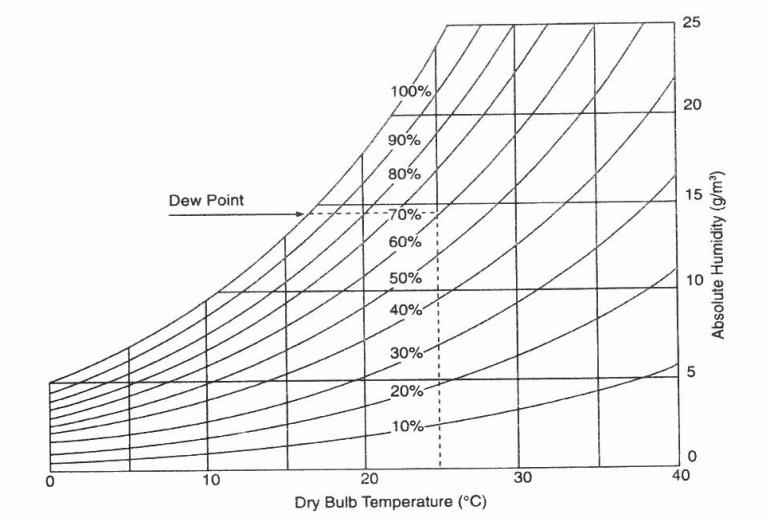
Figure 3: Hygrometric chart (adapted from Thomson 1986).
The hygrometric chart above (Figure 3) illustrates that a relative humidity of 60 % in a well-sealed display case kept at 25 °C will rise to about 80 % relative humidity if the temperature falls to 20 °C. Dew point will be reached if the temperature falls to about 16 °C. Condensation will then occur.
Deterioration Effects
Damage caused by either changes in temperature and relative humidity or by exposure to inappropriate levels of these agents may be either chemical, physical or biological in nature (Figure 4). The main impacts of temperature, unless they are extreme enough to freeze or melt objects, are on relative humidity and the rate of chemical deterioration of objects. A 10 °C rise in temperature for example, approximately doubles the rate at which chemical reactions proceed.
Figure 4: Tar creep induced by warm gallery temperatures.
After light, relative humidity is the most significant factor to be considered in the environmental control of collections. Constant relative humidity above 70 % can cause mould growth and increase corrosion whereas relative humidity levels below 40 % may cause sensitive materials such as paper, parchment and textiles to become brittle.
It is important not only to control relative humidity levels but also to minimise fluctuations. Large and rapid changes in relative humidity caused by sudden temperature variations can have significant effects on materials. A sudden drop in temperature in a display case for example, may result in the dew point being reached. The subsequent condensation will accelerate metal corrosion and encourage biological attack on susceptible organic materials.
Organic materials such as paper, wood, textiles, bone and ivory expand and contract as they absorb and release water in response to changes in relative humidity levels. Rapid fluctuations can lead to cracking and warping of these materials and also cause bonded materials to separate. Paint for instance, may craze or peel from wooden surfaces (Figure 5) and paper glued to a backing board may buckle.
Figure 5: Paint damage due to differential expansion of the underlying wood and the paint layers.
Temperature and Relative Humidity Guidelines
While temperatures in museum display spaces are often designed for visitor comfort rather than for object preservation, conditions in storage areas are usually more carefully defined and controlled (see recommendations for particular material types in other chapters).
For many years the recommended ideal temperature and relative humidity conditions for museum collections were specified as 20 °C and 50 % respectively. These conditions, that were experientially based rather than scientifically determined, are difficult to maintain unless expensive air conditioning systems are used and may not be possible or even desirable in certain areas. In the tropics for instance, where the average yearly relative humidity is about 65 %, it may be better to have this as the optimum level (combined with air circulation) whereas in an arid region it may be better to aim for a relative humidity range of 40 - 50 %. This not only saves on energy costs but also means that material which is conditioned to the ambient relative humidity will not be damaged by change. The following temperature and relative humidity ranges were recommended, on a daily basis for particular climatic zones (Heritage Collections Council 2002):
- hot, humid climates, 22 – 28 °C, 55 – 70 %
- hot dry climates, 22 – 28 °C, 40 – 60 %
- temperate climates, 18 - 24 °C, 45 – 65 %
Storage and/or display of objects in appropriate environments contribute significantly to their longevity. The key is to determine just what the most appropriate environments are for the objects under consideration. Most of the uncertainty with the specification of relative humidity conditions is associated with organic and mixed media materials whereas the conditions for inorganic materials like metals and ceramics are generally more clearly defined.
Since the early 1990s, many scientific studies have been devoted to determining the most appropriate environmental conditions for the storage and display of objects. Early work by Michalski (1993) and Erhardt and Mecklenburg (1994) suggested that although certain material types benefit from storage in strictly controlled conditions, most mixed materials in sound order only need be maintained in environments within a relative humidity range of 40 - 70 %. A relative humidity fluctuation of ± 5% was suggested for sensitive objects (Michalski 1993).
Continued study in this area further refined relative humidity guidelines, with relative humidity variations within the range 30 – 60 % then considered mechanically safe for general collections (Erhardt et al, 2007). More stable conditions must be maintained however for certain degraded objects (veneers and inlays etc) and where possible, lower relative humidity conditions should be maintained for most metal objects. In 2014 following much debate, the Australian Institute for the Conservation of Cultural Materials (AICCM) recommended the following conditions as ‘interim’ guidelines for general collection materials:
- Temperatures to be maintained within the range 15 – 25 °C, with a maximum variation of ± 4 °C in any 24 hour period.
- Relative humidity levels to be maintained within the range 45 – 55 % with a maximum variation of ± 5 % in any 24 hour period.
In addition, the AICCM also recommended that relative humidity control be managed carefully to ensure that where conditions vary seasonally, the relative humidity for collections be maintained within the range of 40 – 60 %.
These more relaxed guidelines were determined after considering the possible impact these changes may have on the preservation of general collections, the need to make collection care more sustainable (especially in light of climate change) and to reduce the carbon footprint and high costs associated with the maintenance of tighter collection conditions. This latter point is significant as previous guidelines were difficult to maintain in most situations without the use of expensive, high energy air-conditioning systems. Similar guidelines to those of the AICCM were also recommended by the American Institute for Conservation (AIC) and endorsed by the International Institute for Conservation of Historic and Artistic Works (IIC) and the International Council of Museums Conservation Committee (ICOM-CC). In fact these latter groups recommended that the ‘interim’ guidelines recommended by the AICCM and the AIC should not be considered as interim but as guidelines per se. Further, the IIC and ICOM-CC groups also recommended that collection care should be achievable for local climates and that consideration should be given to more use of passive methods for environmental control, to the use of simpler technologies, air circulation and lower energy systems.
Conservation management of environmental parameters has also changed somewhat, tending to move away from the specification of strict guidelines (apart from those for particular material types such as acetate films, weeping glass etc) to the adoption of a risk analysis approach. Risk analysis requires an examination of the relationship between the environment and the objects in that particular environment. If the objects are stable within their usual environment then there is little point in altering those conditions. Thus instead of blindly following the strict temperature and relative humidity guidelines specified by many of the larger cultural institutions, anecdotal evidence suggests that it may be better to try to maintain the local conditions to which objects have become acclimatised. This is, of course on the proviso that an appropriate investigation has revealed that these conditions have not caused damage to sensitive objects.
In conjunction with determining the most appropriate relative humidity set point, it is most important to attempt to reduce diurnal and seasonal fluctuations. It is well known that the smaller the fluctuations, the lower the risk of physical damage to susceptible objects. Depending on the chosen relative humidity set point, say 55 %, a variation in daily relative humidity of 5 % from that value would be considered safe for hygroscopic collections, a variation of 10 % would pose a low risk to most organic materials while variations of greater than 20 % would constitute a significantly increased risk to those types of collections.
For most materials, as long as the conditions return to a value close to the original, extreme short-term fluctuations may not be a problem as there is not enough time for objects to respond. Very thin objects however, are much more susceptible to damage from short-term fluctuations than larger more massive objects. These latter objects can take up or lose more moisture without physical impacts becoming evident than can smaller moisture sensitive objects.
Note that for objects containing more than one material type, the relative humidity level of the storage environment should reflect the recommended conditions for the most sensitive component.
Measurement
Relative humidity may be measured by using any of the following devices (Figure 6):
- sling psychrometer;
- thermohygrograph;
- hair hygrometers;
- calibrated electronic devices which provide a digital readout of temperature and RH; and
- data loggers linked to relative humidity sensors
Figure 6: Temperature and relative humidity data loggers (front left), sling psychrometer (front right) and thermohygrograph (rear middle).
One of the simplest instruments for measuring relative humidity is the sling psychrometer. This device is also known simply as the ‘sling’ or as a whirling hygrometer. It consists of two matched thermometers mounted side-by-side, one of which has a fabric sleeve covering it. The end of this sleeve is inserted into a reservoir which is filled with distilled water. The fabric-covered thermometer is known as the wet bulb, the other the dry bulb. When the thermometers are swung around, the water in the sleeve of the wet bulb evaporates, making it cooler than the dry bulb. The amount of evaporation and subsequent cooling depends on the amount of moisture present in the air - the drier the air the greater the level of cooling and vice versa. The difference in the temperatures of the thermometers therefore indicates how dry or humid the air is - the greater the difference the lower the ambient relative humidity, the smaller the difference the higher the relative humidity. A standard hygrometric chart, which displays a series of wet and dry bulb temperature differences and corresponding dry bulb temperatures, is then used to give an accurate measure of the relative humidity.
The sling is used to calibrate many other types of hygrometers. Coupled with a thermohygrograph (seven-day or one-month) an accurate day-to-day or hour-to-hour record of temperature and humidity can be obtained all year round. An advantage of a thermohygrograph is that the recent temperature and relative humidity history of the space being monitored is immediately visible on its chart.
Electronic instruments are also available which record changes in temperature and relative humidity. These devices vary greatly in price and can be obtained from electronic shops or conservation suppliers. These instruments have certain advantages. For example, they may be placed relatively unobtrusively in display cases or in small storage areas in which a thermohygrograph either would not be appropriate or would not fit.
Other relative humidity sensors linked to data loggers can be programmed to record temperature and relative humidity conditions at regular intervals over periods of many months. These sensors are very small and by operating continuously over the different seasons allow useful long-term profiles to be established of storage and display conditions.
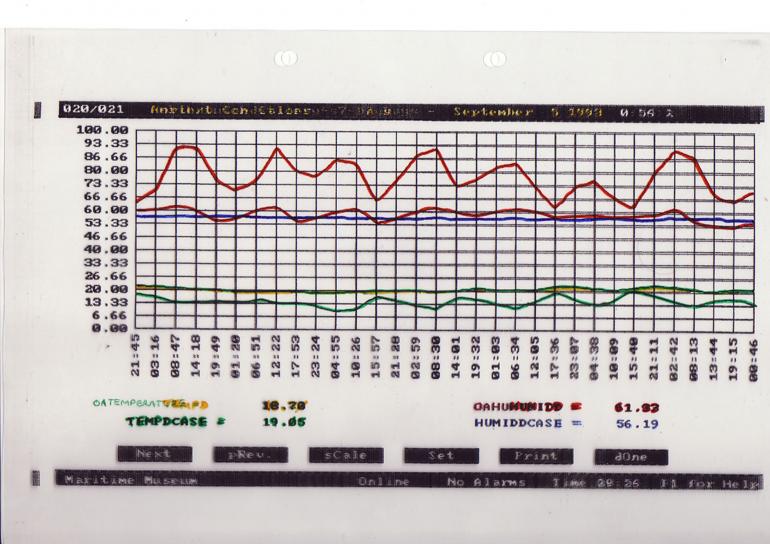
Monitoring relative humidity is important to determine both the actual levels and the fluctuation rates. This information may be used to see how well a building buffers the external ambient conditions and also to see how well a display case further buffers the gallery environment (Figure 7). If the temperature is steady then the relative humidity within a well-sealed display case will remain constant.
Controlling Relative Humidity and Temperature
Relative humidity and temperature control strategies include the use of:
- the buffering effects of buildings and storage media;
- moisture-absorbing desiccants (silica gel and zeolites);
- natural moisture-absorbing materials such as wood, paper and textiles;
- refrigerant dehumidifiers;
- air conditioning systems; and
- thorough and well-planned building maintenance.
We strongly recommend the use of passive methods for temperature and relative humidity control as these are often more sustainable and cost-effective. Appropriate design of buildings and storage media, the use of insulating materials and good management practices are critical components of passive environmental control. These methods are much preferred to more costly and often less reliable air conditioning systems.
Fluctuations in temperature and relative humidity are caused by daily and seasonal fluctuations in the local weather. Even without air conditioning, the insulating effect of a building ensures temperature and relative humidity variations inside a building are generally smaller than those outside. The conditions in the innermost rooms will be the most stable, outer rooms and lofts the most variable and basements the most vulnerable to the development of high relative humidity levels. Thermal insulation of a building will assist in maintaining more stable conditions. The provision of shading (north side of a building in the southern hemisphere) and the use of reflective building surfaces will also assist in reducing the impacts of exterior conditions on interior environments.
Cupboards, boxes and display cases are secondary insulating barriers which provide an additional buffer, helping to stabilise conditions even more (Figure 7). It is interesting to note that the books in a library provide a significant amount of the buffering of the internal library environment.
Figure 7: Temperature and relative humidity readings showing the differences between external conditions, those in an exhibition gallery and in a display case. The uppermost readings highlight the strong buffering of relative humidity inside a display case.
Depending on the internal and external climatic conditions, careful ventilation of a building can also be used to adjust the internal relative humidity. Accurate measurement of the respective relative humidity levels (interior and exterior) is essential for this strategy to be successful.
The climate within an individual showcase or cabinet can be controlled if material particularly sensitive to high relative humidity levels is to be stored or displayed. If the relative humidity is too high (above 65 %) then it may be necessary to use a desiccant within the display case to absorb excess water vapour. Orange self-indicating silica gel can be used for this purpose. Do not use the blue self-indicating silica gel as the cobalt chloride colouring is considered to be carcinogenic. It is important that any silica gel used in display cases is conditioned to the desired relative humidity level prior to being placed in the storage or display space. This process is best left in the hands of conservators as there is a risk of too much moisture being absorbed, resulting in desiccation of vulnerable objects.
Commercial conservation materials such as Art Sorb are also available. They are very useful in helping to control relative humidity levels in display cases and similarly sized containers. Art Sorb is a silica-based material, pre-conditioned to relative humidity levels of either 40, 50 or 60 %. Art Sorb is available in pellet, sheet or cassette form, depending on the nature of the space in which it is to be used. Sheets are generally only available buffered to 50 %.
Zeolite pellets may be of use in climatic areas in which the endemic relative humidity is naturally high (Australian Library and Information Association 1989). A Japanese researcher has developed a treated natural zeolite pellet capable of absorbing and releasing large amounts of water vapour. The pellets have been combined with Japanese paper with the resultant material being produced as a sheet or a paper-covered honeycomb board filled with pellets. Incorporating these materials in storage and display cabinets will minimise relative humidity fluctuations.
Zeolites have the added advantage of being able to absorb odours and as such, have been manufactured and sold commercially in packet or bag form for use in the home. They have been used to lower the relative humidity and absorb odours in refrigerators. If contemplating the use of these products always check their effect in the space in which they are to be used. This is important so that the risk of desiccation, or even inadequate dehumidification, is minimised.
An alternative way of controlling relative humidity levels is to incorporate other materials that absorb moisture, such as treated wood, paper and textiles in the cabinet or display case with the moisture-sensitive artefacts. These water-absorbing materials reduce relative humidity fluctuations by taking up or releasing moisture as conditions change. In this way the artefact is subjected to smaller relative humidity variations. Materials used in this way must be compatible with the moisture-sensitive object. It would not be appropriate for instance, to store a lead object in an oak box or to use alkaline-buffered acid-free tissue paper with leather objects.
In Japan this type of approach is used to deal with the naturally high humidity experienced in that country. Objects are stored in wooden chests in wooden buildings to take advantage of the natural moisture-absorbing and releasing properties of wood. In this way the box contents are buffered against both the naturally high relative humidity and against any changes in external conditions. The relative humidity range inside a Karabitsu (lacquered Japanese cedar chest) for instance, ranged from 60 – 65 % compared to the ambient conditions of 42 – 80 % (Kikkawa and Sano 2008). This approach would not be suitable for all material types however, especially those sensitive to the acidic vapours given off by wood. With the right choice of wood (hoop pine for example) and wrapping of artefacts in acid-free tissue, this approach may allow some of the problems associated with endemically high relative humidity conditions to be overcome without the need for more costly and active intervention.
Alternatively, if passive methods such as those outlined above are not sufficient, this problem may be tackled by lowering the relative humidity of the storage or display area itself. This can be achieved by employing a thermostatically controlled refrigerant dehumidifier to remove excess moisture. As mould formation is encouraged by conditions of high relative humidity, warm temperatures and stagnant air, it may be necessary to employ a combination of dehumidification, air circulation and temperature control. The use of fans within a room will assist in preventing the establishment of ‘dead’ spots in which localised high relative humidity microenvironments may otherwise be established.
Refrigerated air coolers can remove moisture from a building or room by condensing it outside. Water collected by this process is pure enough for use in the sling psychrometer.
Air conditioning systems should not be regarded as the first step in stabilising environmental conditions. While they are very good at maintaining appropriate temperatures, their impact on relative humidity control is highly variable, with higher fluctuations often being recorded following the installation of air conditioning systems. This is particularly the case when air conditioning operates intermittently. If the aim of air conditioning is to maintain objects in the best possible conditions then the system should run for 24 hours a day. If the air conditioning is turned off overnight the environment inside the building will tend towards external values. In winter a lowering of temperature in a well-sealed building will result in an increase in relative humidity. When the air conditioning plant is turned on in the morning there will be a rapid increase in temperature accompanied by a rapid decrease in relative humidity. These rapid changes in relative humidity should be avoided. It is obviously better to use passive controls to minimise fluctuations in environmental conditions.
Air conditioning systems which incorporate humidity control are available but these tend to be very expensive to set up, run and maintain.
It is important to prevent the development of extreme conditions of temperature and relative humidity. Usually such extremes are localised and often only affect a few objects. For example, localised heating and consequent low relative humidity may be caused by direct sunlight falling on an object, by having spotlights positioned too close or by having radiators or heaters adjacent to an object. It is also important to position sensitive objects away from the incoming air stream of air conditioning systems. This allows the incoming air to mix and equilibrate with the bulk of the air in the room before reaching a sensitive object, thereby minimising the impact of relative humidity fluctuations.
A common cause of high humidity is leakage of rainwater through the roof or walls. It is wise not to place objects or shelving against external walls as damp and localised extremes of both temperature and relative humidity are likely. Common sense and adequate building maintenance will minimise such problems.
Biological Pests
Although a chapter in this book is devoted entirely to biological pests a few of the major points are discussed briefly in this section.
Biological pests include moulds, bacteria, insects and other small animals such as mice, rats and birds. All of these organisms are capable of causing severe damage to objects in a collection.
Problems can be minimised by adopting appropriate housekeeping practices and ensuring that the building in which objects are housed is well maintained. Some of the practical ways in which this can be achieved include:
- keeping storage and display areas clean;
- regularly inspecting objects in the collection;
- ensuring that new objects are not infested or contaminated before they are added to a collection;
- blocking access to ceiling spaces by nesting birds, possums and other animals;
- controlling populations of mice, rats and insects by the use of screens, traps and the careful application of residual pesticides;
- keeping food sources away from objects; and
- controlling the environmental conditions so that mould growth and insect populations are not encouraged (keep the relative humidity below 65 %).
Note that trapping rodents is preferable to poisoning them. Trapping allows the rodent’s body to be disposed of appropriately, while poisoning means the body usually ends up as a source of food for some other pest.
Pollutants
Much of the information contained in this section is summarised from lecture notes (2006) and a publication by Jean Tétreault (2003).
Pollutants are compounds, either natural or man-made, that have damaging effects on cultural materials. They may emanate from:
- the materials associated with a collection, including storage and display containers and construction materials;
- the objects in the collection themselves; and
- the surrounding air.
Depending on the type of pollutant and the susceptibility of material types, pollutants have a variety of detrimental effects on objects including increased corrosion of metals and degradation of organic materials. Acetic and formic acids from wood products actively corrode lead and produce efflorescence on calcareous materials such as sea shells, corals and calcium-rich fossils. Ammonia gas produced from alkaline-type silicone sealants, concrete and cleaning products, induces blemishes on ebonite, efflorescence on cellulose nitrate and discolouration of photographic prints and artists’ colourants.
Some pollution-sensitive materials and the airborne pollutants that affect them most seriously are listed below (after Tétreault 2006).
| Sensitive Material | Most Harmful Pollutant |
|---|---|
| Lead | acetic acid |
| Silver | hydrogen sulphide |
| Polyurethane magnetic tapes | water vapour, particles |
| Cellulose acetate | water vapour, acetic acid |
| Cellulose nitrate | water vapour, nitrogen oxides |
| Natural rubber | ozone |
| Colourants (alizarin crimson, basic fuchsin curcumin etc) | nitrogen and sulphur dioxides, ozone |
| Photographic gelatin, colour photo prints | water vapour |
Storage and Display Materials
The most common sources of pollutants in storage areas are the materials used to store and/or display objects. To gain the greatest buffering effect against external environmental changes, it is desirable for objects to be stored in well-sealed containers. This is problematic however, unless the materials used in these containers are stable, of archival quality and neither they nor the object to be placed in the container emit harmful pollutants. Otherwise pollutant levels will build up to damaging levels in the container. Materials which are known to release harmful vapours at room temperature (modified after Padfield et al 1982) include:
- wood (particularly hardwood and composite boards);
- protein-based glues (except photographic gelatin);
- cellulose nitrate and cellulose acetate;
- highly plasticised polyvinyl chloride;
- polyvinyl alcohol;
- RTV silicones;
- polyurethane;
- rubbers containing sulphur vulcanising agents;
- wool and felt;
- some dyes which contain labile sulphur; and
- concrete
Materials which are considered to be ‘safe’ (modified after Padfield et al 1982) include:
- metals;
- ceramics and glass;
- inorganic pigments;
- polyethylene and polypropylene;
- acrylic paints and varnishes;
- polycarbonates;
- polystyrene and modified polystyrene such as high impact polystyrene and acrylonitrile/butadiene/styrene (ABS);
- polyesters and fibres including polymethyl methacrylate (Perspex, plexiglass), polyethylene terephthalate; and
- unbleached and undyed cotton and linen.
Materials such as chipboard, wood, certain polymers and paints can contribute to the deterioration of objects in a collection. Oak and cedar for example give off high amounts of acetic acid, promoting the corrosion of nearby metal objects, with lead objects particularly susceptible (Figure 8). It has been demonstrated that carbonyl pollutants such as acetic acid from wood are more prevalent in internal museum environments than in the external air with concentrations increasing in the following order:
Galleries < storage areas < display cases < storage cabinets
Data of this sort provides clear evidence of the use of inappropriate materials and the build-up of pollutants in more confined spaces.
Figure 8: A lead painted model battleship showing corrosion induced by acetic acid vapours given off by wood used in display case construction.
Objects in direct contact with certain polymer storage files may become contaminated as plasticisers migrate from these polymers. Artefacts stored in highly plasticised polyvinyl chloride are particularly at risk owing to the migration of plasticisers and the possible release of corrosive hydrogen chloride from this polymer. Staining is likely for objects in contact with acidic papers and some paints and adhesives while malleable materials like plasticine and blu tack will transfer sulphur and oils respectively to any objects with which they come into contact.
The placement of objects in freshly painted wooden cupboards or rooms is also not recommended as the solvent fumes from the drying paint may affect the organic components of some objects (glues, resins, plastics, lacquers and the like). It is recommended that a newly painted, internal surface be allowed to cure for at least four weeks before use (see Appropriate Materials later in this section for additional recommendations). Use paints that contain low levels of volatile organic components.
Artefacts
The objects themselves may prove to be pollutant sources as they degrade. Cellulose nitrate movie films for example, not only release acidic oxidising gases but also pose a fire risk as they deteriorate. These materials must be stored in their own well-ventilated and preferably fire-proof area.
This in-built, intrinsic form of pollution is often much harder to deal with than either airborne pollutants or pollutants that migrate from materials in contact. It is most important therefore to understand the nature of the materials from which collection objects are made so that precautions can be taken for those with a pre-disposition to deterioration.
Airborne Pollutants
Airborne pollutants including dust, oxides of nitrogen and sulphur, hydrogen sulphide, peroxides, ozone, cooking fumes and airborne salt may arise from vehicle emissions, industrial activity or decaying organic matter.
Dust can be made up of a variety of materials including fine dirt, salt particles, oily mists, textile fibres and wood and metal powders. The precise nature of the dust and the objects on which it settles will determine its effect. Dust can promote both chemical and biological attack on objects. It is particularly damaging for magnetic media such as polyurethane sound and visual recording tapes. Dust causes damage to objects by:
- physical abrasion and discolouration;
- attracting moisture; and
- attracting biological pests such as insects, mould and bacteria.
The deposition of small particles on objects often initiates deterioration processes. Metal corrosion often starts after particles are deposited on a surface, oily residues can absorb potentially damaging pollutants and many deposited salts are hygroscopic. Finer particles are generally more damaging than coarser particles. Finely divided dust is harder to clean, particularly from fragile or complex porous surfaces and increases the risk of damage to an object during cleaning. Note that unsealed concrete is a source of alkaline dust.
A further issue is the perception that dirty objects create. If objects on display are coated with dust it gives visitors the impression that the collections are not being well cared for.
The amount and type of dust can be monitored quite simply by using standardised ‘sticky’ surfaces such as double-sided tape stuck to a glass microscope slide. After exposure these dust traps should be covered with a microscope cover slip to stop further dust accumulation prior to optical microscopic examination. Classification of particles (e.g. salt, soil, clothing fibres, soot etc) and estimations of quantities will allow remedial action to be taken to reduce further dust deposition.
Note that for some objects, water vapour and oxygen in the air must also be considered to be pollutants as they can cause material deterioration in susceptible artefacts.
Preventive Measures
The significance of any air pollution problem will be determined largely by the particular collection environment and its location, be it in an industrial, tropical, coastal or desert site. Each particular environment will have its own set of problems. Pollutants may be transported by moving air or by direct contact between objects which allows the transfer of damaging materials from one object to another.
Although reducing pollutant levels is not easy, preventive measures will help to reduce damage to objects. Preventive action may involve combinations of the following:
- good housekeeping;
- exclusion of pollutants;
- sealing of exposed surfaces such as concrete and timber walls and floors;
- the use of archival quality storage and display materials;
- incorporation of absorbent materials in storage containers;
- appropriate use of ventilation and air exchange;
- reducing chemical reactions by reducing relative humidity, temperature, light and oxygen; and
- using filtered air conditioning.
The Building and Storage Enclosures
As with buffering against changes in relative humidity, the first line of defence against externally generated pollutants is the building itself. As a rough rule of thumb and, as long as there are no significant indoor pollution sources, if the pollutant level outside is 100, it will be approximately 10 inside a room and 1 inside a box (Tétreault, 2003).
A simple air-tight enclosure, containing no noxious substances, is therefore the simplest way of isolating an object from damaging pollutants. Valuable objects that deteriorate in the presence of oxygen can be sealed in an air-tight container filled with an inert gas like argon. Note that the argon should be refilled on a regular basis. If an object is to be stored in an airtight container it is critical that neither the object nor the container are potential sources of damaging emissions and that relative humidity fluctuations are controlled within the container. It would be most unwise for instance, to store cellulose acetate and cellulose nitrate objects or acidic paper in well-sealed enclosures.
If objects are stored in boxes, it is a good idea to make a mylar ‘window’ in the box so that the object can be viewed without having to open it. As monitoring of an object’s condition is an important part of its on-going preservation, the presence of ‘windows’ in stacked boxes is invaluable, as are regular inspections of objects.
When considering the use of airtight enclosures take relative humidity and pest control factors into account.
Appropriate Materials
Boxes and supporting materials for objects should usually be made of archival quality materials. This may not be necessary for all objects however. If acidic newspapers are to be stored in cardboard boxes for example, the use of acid-free boxes will be much more costly and of little long-term benefit with respect to minimising acid development in the paper. Slightly acidic cardboard boxes could be used as long as the storage environment has a stable relative humidity (approximately 50 %) and the boxes are not exposed to direct wetness. See other chapters in this book and consult a conservator if in doubt about the type of materials that are best suited for storage enclosures for particular material types.
Use enamelled, galvanised or powder coated metal, stainless steel, acrylic or glass cabinets and shelves in preference to wood or chipboard for storage and display, particularly for enclosed spaces.
Wood and wood products should be avoided for storage containers, cupboards and display cabinets, especially for acid-sensitive objects. Some timbers, plywood and composite board products may be used however, depending on the type of wood, the presence and type of surface coatings, the type of resin/glue used in manufacture and/or the presence and type of laminating surfaces. Some examples of wood products suitable for storage and display systems include (after Tétreault 2006):
- plywood with phenol formaldehyde impregnated paper overlays (high density overlaid);
- plywood with ABS plastic laminates;
- plywood with phenolic laminates (e.g. Arborie, Formica);
- exterior grade plywood; and
- particle board without urea formaldehyde resins.
If laminated wood products are used for storage systems it is critical that all surfaces are completely sealed, particularly the ends and edges. As a general principle exterior grade products emit less damaging pollutants and are of better quality than similar products designed for interior use.
It is strongly recommended that if there is no alternative other than to use products such as plywood, chipboard or medium density fibreboard then all surfaces should be either laminated (as described above), coated with plastic or aluminium barrier foils or primed and painted with at least 2 top coats. Although this latter method will minimise emissions, pollutants released by the wood products will eventually diffuse through the paint or varnish layers. Oxidative paints, including oil-based polyurethane, alkyds and epoxy esters (epoxy in one can) should not be used to paint wooden surfaces into which artefacts are to be placed. Other paints, including two-pack epoxies and water-based polyurethanes are acceptable, subject to appropriate drying periods before use. Varnishes and water-based paints are not as effective as polyurethane lacquers as a coating barrier. If coated wood products are used for open shelving then four days drying time is sufficient. In enclosed spaces however, four weeks drying time is essential.
Ventilation, Sorbents and Filters
If it is not possible to avoid pollutants either by using archival quality storage materials or airtight enclosures then alternative strategies must be adopted. These may include the use of appropriate ventilation, sorbents or filters.
Sorbents effectively ‘soak up’ pollutants from the environment. They are generally very good at mitigating the impacts of externally generated pollutants and will therefore protect objects in well-sealed containers. They are not as good at protecting objects against high levels of internally generated pollutants (such as off-gassing from wood) as the sorbent will quickly become saturated. Ventilation may be a better option in the latter case.
Sorbent materials include activated carbon, activated charcoal cloth, zeolites, silica gel, activated alumina and specifically impregnated composites. Each of these materials has specific absorption characteristics and none of them will control all possible pollutants. Activated charcoal cloth for instance, is very good at absorbing acetic acid vapours but has minimal effect on hydrogen sulphide or nitrogen oxides. Some sorbents and the pollutants that they are most effective against are summarised below (after Tétreault 2003).
| Sorbent | Most effective against |
|---|---|
| Activated carbon | acetic acid, ozone, sulphur dioxide |
| Activated charcoal cloth | acetic acid, ozone |
| Activated alumina (with potassium permanganate) | acetic acid, hydrogen sulphide, ozone, sulphur dioxide |
| Molecular sieves (zeolite) | water vapour |
| Silica gel | water vapour |
| Zinc oxide | hydrogen sulphide |
A variety of sorbents are available that incorporate different impregnating materials. These composite sorbents have the ability therefore to control more potential pollutants. For example, in addition to having good sorption properties for acetic acid, ozone and sulphur dioxide, activated carbon containing potassium carbonate is also good at absorbing hydrogen sulphide and nitrogen dioxide.
A sorbent may be incorporated as a layer or liner in storage and display areas or even as a wrapping material around a sensitive object (as long as the object does not have to be visible). Although expensive, activated charcoal cloth can last up to ten years, depending on the level of pollution. Commercially available Microchamber™ papers and boards incorporate zeolites and activate charcoal and are capable of providing protection against both acidic and oxidising pollutants such as ozone, oxides of nitrogen and sulphur dioxide. These products afford much greater protection to artefacts than alkaline buffered boards and papers.
An often overlooked factor in controlling pollutant effects is the importance of relative humidity control, both to reduce its impact on susceptible materials (for which it must be considered to be a pollutant) and also to lower the rates of other pollutant-induced chemical reactions. Metals which are more prone to corrosion at elevated relative humidity levels benefit from storage or display in low relative humidity environments. Implement strategies as outlined in the earlier Relative Humidity and Temperature section of this chapter for those objects that can tolerate lower relative humidity conditions.
The effects of people and their clothing should also be considered when reviewing pollution sources. Bio-effluents (colloquially the fart), off-gassing, particularly from wet clothing and the loss of skin and cloth fibres can contribute to deterioration in susceptible objects in poorly ventilated spaces. Having a separate area for visitors to leave coats and limiting the number of visitors will reduce potential pollutants and increase visitor comfort.
Dust Control
Clean areas regularly and maintain buildings to minimise dust problems. Practical steps that can be taken to reduce dust in collections include the following:
- clean storage and display areas with a good quality vacuum cleaner (HEPA filter recommended) rather than a broom. This reduces the amount of dust that may be spread and/or resuspended during cleaning;
- use a damp cloth rather than a feather duster for exposed surfaces;
- use dust covers, closed containers and/or covered shelving to protect artefacts from dust contamination; and
- maintain building surroundings to minimise dust generation.
For objects in storage, protective wrapping with materials such as washed cotton or linen and/or acid-free tissue will provide protection against dust and some pollutants.
For museums, keeping a one metre gap between visitors and objects will result in approximately 90 % of human-generated dust (cloth fibres, human dander etc) being deposited before it reaches the objects. This is because larger dust particles drop closer to their source. Finer particles remain suspended in the air for longer periods and can be transported further by air circulation in a building.
Air Conditioning
Larger institutions, museums and art galleries often rely on heating, ventilation and air conditioning (HVAC) systems to maintain internal conditions that are both comfortable for visitors and appropriate for preservation of objects on display. In addition to providing basic temperature and relative humidity control HVAC systems can also improve air quality by filtering out particles and gaseous pollutants. As there are many different HVAC systems, filters, components and designs, consultation with specialist engineers is essential in order to match a system to a particular environment, building and its contents.
Sophisticated HVAC systems are expensive to set up, operate and maintain. Less costly alternatives, while not as effective, include the use of using portable filtration units in rooms and the careful placement of objects to reduce exposure to pollutants (away from open windows etc). Increased ventilation may help to dilute internally generated pollutants while restricted ventilation will help to exclude externally produced pollutants. The strategy to be used must be determined by a careful examination of the situation in question.
While the majority of this section has concentrated on the effects and alleviation of airborne pollutants, the impact of pollutants that migrate via direct contact must also be taken into account. Avoid direct contact between objects and coatings for instance. Archival lining materials such as acid-free paper or board, polyethylene, polypropylene, polyethylene terephthalate and mylar can be used to separate objects from shelving or other materials from which contaminants may migrate. Such separation may even be as simple as sealing in a polyethylene bag.
Monitoring of collections is an important part of on-going collection care. While it is desirable to be able to monitor pollutant levels in storage and display areas, this requires specialised equipment and is often costly to undertake. In reality, what often happens is that the objects themselves become the deterioration monitors - when an object deteriorates, the nature of its deterioration gives an indication of the likely cause, providing a guide to appropriate follow-up remedial action. The key is to regularly inspect objects in collections to ensure that preventive action can be taken as soon as possible.
Summary
- Know the material types and the agents of decay that are most likely to cause damage in a collection so that appropriate steps can be taken to minimise deterioration and damage.
- Exclude direct sunlight from storage and display areas.
- Maintain light, temperature and relative humidity levels in storage and display areas that are best suited to minimise the deterioration of the most sensitive objects in those areas.
- Use archival quality materials to store and display artefacts.
- Where possible apply passive, low technology techniques to buffer changes in relative humidity and temperature rather than rely on expensive mechanical systems.
- Isolate artefacts that are prone to deterioration from more stable artefacts.
- Focus on improving storage and display conditions than applying treatments to deteriorated or damaged artefacts.
- Ensure that house-keeping standards are high and that buildings are well maintained so that the effects of biological pests are reduced and internal environmental conditions are as good as they can be.
- Regularly monitor the condition of stored artefacts.
Bibliography
Artcare, The Care of Art and Artefacts in New Zealand, 1998, Auckland Art Gallery Toi o Tāmaki, Auckland, New Zealand.
Australian Library and Information Association, 1989, Fumigation, Conservation of Library Materials, Newsletter No. 5, p. 3.
Bachmann, K., (Ed.), 1992, Conservation Concerns: A Guide for Collectors and Curators, Smithsonian Institute Press, Washington.
Caple, C., (Ed.), 2011, Preventive Conservation in Museums, Routledge, London and New York.
Grattan, D. and Michalski, S., 2009, Environmental guidelines for museums – temperature and relative humidity (RH), www.cci-icc.gc.ca/crc/articles/enviro/controls-niveaux-eng.aspx
Erhardt, D. and Mecklenburg, M., 1994, Relative humidity re-examined, in Preventive Conservation: Practice, Theory and Research, Preprints of the Contributions to the Ottawa Congress, September, 1994, IIC, pp. 32-38.
Erhardt, D., Tumosa, C.S. and Mecklenburg, M.F., 2007, Applying science to the question of museum climate, in Padfield, T. and Borchersen, K. (Eds), Museum Microclimates. Contributions to the conference in Copenhagen, 19-23 November, 2007, The National Museum of Denmark, pp 11-18 (www.microclimates.natmus.dk).
Guidelines for Environmental Control in Cultural Institutions, 2002, Heritage Collections Council, Canberra, ACT, Australia.
Kikkawa, Y. and Sano, C., 2008, Preservation of Historic Paper in Japan, in Conservation and Access. Contributions to the London Congress, 15 – 19 September, 2008, The International Institute for Conservation of Historic and Artistic Works, p 266.
Michalski, S., 1993, Relative humidity: a discussion of correct/incorrect values, in Preprints, ICOM Committee for Conservation 10th Triennial Meeting, Washington DC, pp. 624-629.
Museums Australia Inc. (NSW), 1994, Museum Methods: A Practical Manual for Managing Small Museums, Museums Australia Inc. (NSW).
Padfield, T and Borchersen, K., (Eds), 2007, Museum Microclimates: Contributions to the Copenhagen conference, 19-23 November 2007, National Museum of Denmark, www.microclimates.natmus.dk
Padfield, T., Erhardt, D. and Hopwood, W.R., 1982, Trouble in store, in N.S. Brommelle and G. Thomson (Eds), Science and Technology in the Service of Conservation, Preprints of the Contributions to the Washington Congress, 3-9 September, 1982, IIC, London, pp. 24-27.
Powerhouse Museum, 1994, Caring for Heritage Objects, Powerhouse Museum Research Series No. 3, Sydney.
Tétreault, J., 2003, Airborne Pollutants in Museums. Galleries and Archives: Risk Assessment, Control Strategies, and Preservation Management, Canadian Conservation Institute, Ottawa, Canada.
Tétreault, J., 2006, Display and Storage Products Used in Museums, Lecture Notes, Canadian Conservation Institute, Ottawa, Canada.
Thomson, G., 1986, The Museum Environment, 2nd Edition, Butterworths, London.