Modern Organic Materials
I. M. Godfrey and I. Loo
Introduction
Plastics and rubbers have played important roles in human endeavours since the beginning of the 20th century, pervading all aspects of modern societies. Their uses range from packaging (the ubiquitous plastic bag), sporting equipment such as surf boards and tennis racquets, information storage media such as tapes and disks, adhesives, paints, textiles and works of art to motor vehicle components. The sheer range of modern plastic and rubber materials, the different forms into which they can be shaped or moulded, from films, foams and fibres to three dimensional objects and their huge range of different physical and chemical properties have ensured their prominence in modern life. This prominence suggests that objects composed of these types of materials will make up an increasing proportion of museum and private collections in the future.
Plastics and rubbers belong to a group of substances known as polymers. Polymers are chemical compounds made up of smaller units (monomers), linked together to form larger structures that may be either natural or synthetic. Examples of naturally occurring polymers include cellulose in wood, collagen in proteins, latex from the para rubber tree (Hevea brasiliensis) and shellac from the female lac bug. The earliest man-made polymers can be considered to be semi-synthetic as they were made by chemically modifying natural polymers.
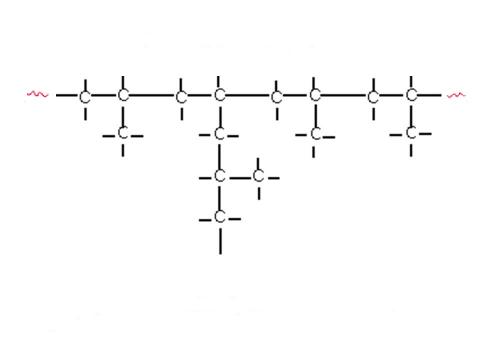
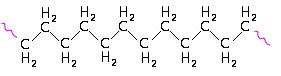
Some polymers form links which result in long chains with very little cross-linkage between the individual chains. Small side-chains or branches may also be present at intervals along the main polymer chain (Figure 1). These substances, known as thermoplastic resins, do not undergo any change during moulding other than a change of shape. They will soften if the temperature is raised, form a rigid solid when the temperature is lowered and tend to be soluble in organic solvents.
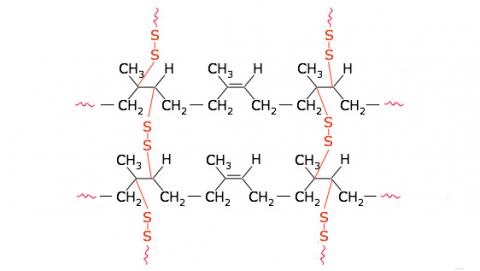
Other polymers form cross-links between long chains, an arrangement which usually leads to the production of a much harder and less soluble polymer than the purely chain-like equivalent. These polymer types, known as thermosetting resins, undergo a chemical change (curing) due either to the effect of heat or a catalyst, which makes them infusible. They do not soften again, even if exposed to heat and tend to be insoluble in organic solvents.
Figure 1: Types of polymers.
(a) Straight chain polymer - polyethylene.
(b) Branched chain polymer – typical structure.
(c) Cross-linked polymer - vulcanised rubber with disulphide cross-links.
The chemical nature of the polymer itself, the length of the polymer chains, the number and type of side chains that branch from the main chain, the presence of cross-linkages between chains and additives such as plasticisers, UV absorbers, fillers, stabilisers, flame retardants and colorants all affect the properties of a particular plastic. High density polyethylene for instance, which has few branches from the main polymer chain is more chemically resistant and stronger than low density polyethylene which has more branches. This is because as the number of branches increases, it reduces the ability of the polymer chains to pack tightly together, thereby reducing the polymer density.
The first polymers developed were those that involved the chemical modification of natural polymers. Cellulose nitrate was first prepared in 1833 (developed as the explosive ‘gun cotton’ in 1846) and rubber was vulcanised in 1839. In this latter process sulphur atoms were used to form cross-links between the natural rubber chains, allowing a new moulding material to be produced with markedly different properties.
Other important milestones included the preparation of a type of thermosetting plastic used in the production of ‘union’ cases in 1853 (see the chapter Photographs), the production of mouldable cellulose nitrate in the 1860s (‘Parkesine’), its subsequent plasticising with camphor in the mid-1860s, the preparation of cellulose acetate in the 1890s (commercially viable by 1910) and that of casein-formaldehyde plastic in 1899.
The first fully synthetic polymer was produced by reacting formaldehyde with phenol. This polymer, developed by J. L. Baekeland in 1907 was dark in colour, hard and brittle (‘Bakelite’). Bakelite dominated the domestic market until the 1950s and was used for items as diverse as toasters, clocks, radios, ash-trays, toilet seats, electrical fittings, car components and even coffins. The subsequent development of urea-formaldehyde and combined urea and thiourea-formaldehyde resins provided an unlimited colour range to complement the rather dark, sombre tones of the phenol-formaldehyde range.
The ‘poly’ age began in the 1930s. Thermoplastic resins began to make an impact with the development of polyvinyl chloride (PVC) and polystyrene. Polymethyl methacrylate (‘Perspex’) was first produced commercially in 1933. Its shatter-resistant properties were soon in demand. The development of polyethylene (‘polythene’) soon followed in the 1940s. The commercial development of polyesters, silicones, polyamides such as nylon 6, 6 and polyurethanes all occurred about this time.
Although the speed and number of developments in the history of polymers preclude any further discussion here, texts are available which document these developments and provide details of major polymer types, physical and chemical properties, preparation and technology (Kaufman 1963, Kroschwitz 1987, Mossman 1997, Shashoua 2008).
This is an area in which there is continuing research and development. The next generation of plastics is being produced by combining resins, both together and with fillers, and reinforcing agents. Advances in electronic and automotive engineering depend heavily on plastics. This, combined with the increasing use of plastics in household appliances, sporting goods, electronic devices and a myriad of other areas will ensure that these objects will be collected, forming the basis of historical and technological collections.
Identification
For collectors the identification of a polymer type is important because it may provide useful information about the origin and authenticity of an object and also about how it is likely to deteriorate if not looked after carefully.
Although a few simple tests have been described which may allow the more common plastics to be identified (Braun 1996, van Oosten 1999, Shashoua 2008), the identification of plastics is not particularly easy. Some plastics such as cellulose nitrate have characteristic appearances (Figure 2) while others have distinctive odours. Cellulose acetate for instance, has a vinegar-like smell, whereas plasticised PVC has a ‘new car’ vinyl odour when warmed under running hot water. The odour of degrading or very new polymers is often informative.
Figure 2: 1930s cellulose nitrate imitation of tortoise shell powder bowl and lid (Copyright: Museum of Design in Plastics, Arts University, Bournemouth, Wallisdown, United Kingdom).
For some polymers it may be possible to use visual clues such as design, colour and trademarks to assist in identification. Function, touch, date of manufacture, flotation, solubility, density and flame tests may also allow a tentative identification to be made. Some of these tests are destructive however and should be undertaken with caution and only after careful familiarisation with the techniques involved. As the number and type of polymers is constantly growing, it is beyond the scope of this book to attempt to describe characteristic features of all commonly used plastics and rubbers. Readers are encouraged to refer to excellent texts such as Conservation of Plastics (Shashoua 2008) for further information.
Where a doubt exists as to the identity of a plastic it may be necessary to use analytical techniques such as Fourier transform infra-red (FTIR) spectroscopy, Raman spectroscopy or combined pyrolysis gas chromatography-mass spectrometry to allow a conclusive identification to be made. Shashoua (2008) provides copies of FTIR spectra of commonly collected polymers. Both FTIR and Raman spectroscopic analyses can be carried out non-destructively. Depending on the nature and amount of additional components present in an object the spectra can sometimes be difficult to interpret. Thus while these techniques will be able to identify at least the major organic component (the polymer) of a plastic, other techniques are more suited to the identification of plasticisers, stabilisers, dyes, pigments and fillers which may also be present in these substances (Shashoua 2008).
In addition to identifying the components of a polymeric substance, analytical techniques can also characterise the extent of degradation of the substance and often the degradation mechanism or mechanisms that are operating. This knowledge is very useful as it can be used to guide decisions regarding conservation and preservation options.