Appendix 9: Spot Tests for Common Metals
Spot tests may be used to identify metals and to distinguish different metals which make up an alloy. Simple instructions and a list of the tests are provided to facilitate identification of a metal. Note that these tests are only qualitative, providing information regarding the components of a metal but not the proportions in which they are present. These tests follow the procedures described by Laver (1978).
General Electrolysis Instructions
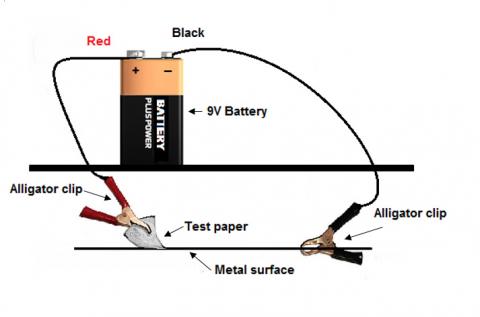
Figure 1: Equipment set-up for metal spot tests (adapted from Laver 1978)
- tests should be carried out in unobtrusive spots on the artefact, as marks may be left;
- remove protective coatings such as lacquers and waxes from the area to be tested, since no reaction will occur otherwise;
- the test papers to be used in conjunction with electrolysis are best cut into triangles. When wet with distilled water or salt solution the paper should be shiny-wet, but not soaking;
- the clip needs to be touched firmly to an area of reasonably solid metal;
- touch the test paper in the tweezers to the surface of the object, about one centimetre away from the clip. Do not let the tweezers touch the metal;
- rinse with distilled water and dry wet spots of electrolyte (sodium chloride for example) or other reagents; and
- disconnect the battery and store it away from the tweezers and clip to avoid accidental discharging if the two should touch.
List of Tests
Antimony (Sb)
Procedure
- dip a small piece of antimony test paper in diluted hydrochloric acid (HCl, 2 %) and apply to the object.
Results
- the presence of Sb is indicated by an orange colour;
- the reaction is complete in five seconds on pure Sb using 2 % HCl. Surfaces containing traces of Sb will be much slower; and
- gold and silver surfaces remain unaffected by the test, lead is slightly darkened, copper and iron corrosion products change colour slightly.
Copper (Cu)
Procedure
- wet a small piece of commercial test paper (Cuprotesmo) with distilled water and place on the surface of the metal.
Results
- the copper metal or Cu+ and Cu2+ ions cause the pale yellow paper to turn pink-purple;
- this test works particularly well on corroded or patinated areas and leaves no trace of the test; and
- on highly polished or new surfaces the reaction is much slower, but may be accelerated by briefly applying the electrode to the paper (about five seconds is sufficient).
Gold (Au)
Procedure
- dip a small triangle of plain filter paper in a saturated aqueous solution of sodium chloride (NaCl);
- electrolyse for less than 15 seconds. Some darkening will probably be evident if copper is present. Leave the paper on the spot until slightly dried to ensure that gold is on the surface of the paper; and
- dip into a mixture of stannous chloride (SnCl2, 20 %) in HCl (15 %).
Results
- the presence of gold causes the paper turn black.
Iron (Fe)
Procedure
- for corroded objects, dip a small square of dipyridyl test paper in distilled water and place on the surface of the object. This leaves no visible effect on the object;
- for uncorroded objects, dip a long piece of dipyridyl test paper in a saturated NaCl solution so that the paper is quite wet;
- electrolyse; and
- the paper is long to prevent confusion with any colour reaction which occurs with the steel of the tweezers.
Results
- for both corroded and uncorroded objects, a red colour will appear on the white test paper after several seconds if iron is present.
Nickel (Ni)
Procedure
- dip a small piece of nickel test paper into a saturated NaCl solution; and
- electrolyse for about five seconds.
Results
- on drying, the following colours may be observed - pink-red for nickel, brown for iron, green for copper and yellow for gold.
Silver (Ag)
Procedure
- wet a filter paper with 10 % potassium chromate (K2CrO4) and touch the point of the triangle to the metal in an area that is as small as possible; and
- electrolyse for one second or less.
Results
- the presence of silver is indicated by the formation of red silver chromate (Ag2CrO4) in the spot on the metal; and
- this mark, if small, can be polished off very easily.
Tin (Sn)
Procedure
- dip a small piece of filter paper in a saturated, aqueous solution of cacotheline (0.6%);
- when the cacotheline dries slightly, dip the filter paper in a saturated solution of NaCl; and
- electrolyse.
Results
- A purple colour indicates tin; and
- shiny surfaces will become matt and slightly darker after two seconds of electrolysis.
Zinc (Zn)
Procedure
- dip a small piece of filter paper in sodium hydroxide solution (NaOH, 5 – 10 %);
- apply the paper to the surface of the object for five to ten seconds;
- electrolysis is recommended;
- when the sample has been absorbed in the filter paper, place this paper in the centre of a larger filter paper, making a wet spot; and
- wash this spot with successive drops of dithizone/carbon tetrachloride*.
Results
- pink (not orange) around the spot edges denotes zinc;
- any NaOH remaining on the metal of the object should be wiped off immediately. This can be done with the same filter paper on which the dithizone reaction is to be done;
- shiny zinc surfaces may be slightly darkened or dulled after electrolysis;
- there is a small effect on some copper corrosion products; and
- a lead surface tends to develop a shiny spot where the sodium hydroxide droplet was.
* Note that carbon tetrachloride is a known carcinogen and this test must be conducted in a fume cupboard.
Reference
Laver, M., 1978, Spot tests in conservation: metals and alloys, in Preprints, The International Council of Museums Committee for Conservation, 5th Triennial Meeting, Zagreb, 1978, vol.3, 78/23/8.