Modern Organic Materials
I. M. Godfrey and I. Loo
Introduction
Plastics and rubbers have played important roles in human endeavours since the beginning of the 20th century, pervading all aspects of modern societies. Their uses range from packaging (the ubiquitous plastic bag), sporting equipment such as surf boards and tennis racquets, information storage media such as tapes and disks, adhesives, paints, textiles and works of art to motor vehicle components. The sheer range of modern plastic and rubber materials, the different forms into which they can be shaped or moulded, from films, foams and fibres to three dimensional objects and their huge range of different physical and chemical properties have ensured their prominence in modern life. This prominence suggests that objects composed of these types of materials will make up an increasing proportion of museum and private collections in the future.
Plastics and rubbers belong to a group of substances known as polymers. Polymers are chemical compounds made up of smaller units (monomers), linked together to form larger structures that may be either natural or synthetic. Examples of naturally occurring polymers include cellulose in wood, collagen in proteins, latex from the para rubber tree (Hevea brasiliensis) and shellac from the female lac bug. The earliest man-made polymers can be considered to be semi-synthetic as they were made by chemically modifying natural polymers.
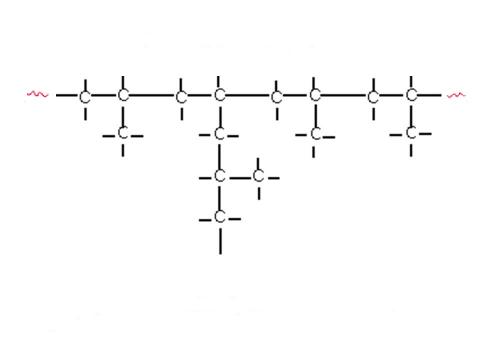
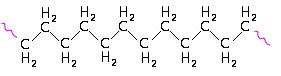
Some polymers form links which result in long chains with very little cross-linkage between the individual chains. Small side-chains or branches may also be present at intervals along the main polymer chain (Figure 1). These substances, known as thermoplastic resins, do not undergo any change during moulding other than a change of shape. They will soften if the temperature is raised, form a rigid solid when the temperature is lowered and tend to be soluble in organic solvents.
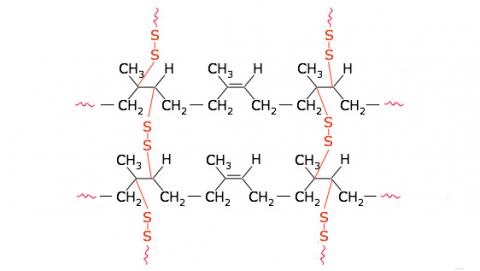
Other polymers form cross-links between long chains, an arrangement which usually leads to the production of a much harder and less soluble polymer than the purely chain-like equivalent. These polymer types, known as thermosetting resins, undergo a chemical change (curing) due either to the effect of heat or a catalyst, which makes them infusible. They do not soften again, even if exposed to heat and tend to be insoluble in organic solvents.
Figure 1: Types of polymers.
(a) Straight chain polymer - polyethylene.
(b) Branched chain polymer – typical structure.
(c) Cross-linked polymer - vulcanised rubber with disulphide cross-links.
The chemical nature of the polymer itself, the length of the polymer chains, the number and type of side chains that branch from the main chain, the presence of cross-linkages between chains and additives such as plasticisers, UV absorbers, fillers, stabilisers, flame retardants and colorants all affect the properties of a particular plastic. High density polyethylene for instance, which has few branches from the main polymer chain is more chemically resistant and stronger than low density polyethylene which has more branches. This is because as the number of branches increases, it reduces the ability of the polymer chains to pack tightly together, thereby reducing the polymer density.
The first polymers developed were those that involved the chemical modification of natural polymers. Cellulose nitrate was first prepared in 1833 (developed as the explosive ‘gun cotton’ in 1846) and rubber was vulcanised in 1839. In this latter process sulphur atoms were used to form cross-links between the natural rubber chains, allowing a new moulding material to be produced with markedly different properties.
Other important milestones included the preparation of a type of thermosetting plastic used in the production of ‘union’ cases in 1853 (see the chapter Photographs), the production of mouldable cellulose nitrate in the 1860s (‘Parkesine’), its subsequent plasticising with camphor in the mid-1860s, the preparation of cellulose acetate in the 1890s (commercially viable by 1910) and that of casein-formaldehyde plastic in 1899.
The first fully synthetic polymer was produced by reacting formaldehyde with phenol. This polymer, developed by J. L. Baekeland in 1907 was dark in colour, hard and brittle (‘Bakelite’). Bakelite dominated the domestic market until the 1950s and was used for items as diverse as toasters, clocks, radios, ash-trays, toilet seats, electrical fittings, car components and even coffins. The subsequent development of urea-formaldehyde and combined urea and thiourea-formaldehyde resins provided an unlimited colour range to complement the rather dark, sombre tones of the phenol-formaldehyde range.
The ‘poly’ age began in the 1930s. Thermoplastic resins began to make an impact with the development of polyvinyl chloride (PVC) and polystyrene. Polymethyl methacrylate (‘Perspex’) was first produced commercially in 1933. Its shatter-resistant properties were soon in demand. The development of polyethylene (‘polythene’) soon followed in the 1940s. The commercial development of polyesters, silicones, polyamides such as nylon 6, 6 and polyurethanes all occurred about this time.
Although the speed and number of developments in the history of polymers preclude any further discussion here, texts are available which document these developments and provide details of major polymer types, physical and chemical properties, preparation and technology (Kaufman 1963, Kroschwitz 1987, Mossman 1997, Shashoua 2008).
This is an area in which there is continuing research and development. The next generation of plastics is being produced by combining resins, both together and with fillers, and reinforcing agents. Advances in electronic and automotive engineering depend heavily on plastics. This, combined with the increasing use of plastics in household appliances, sporting goods, electronic devices and a myriad of other areas will ensure that these objects will be collected, forming the basis of historical and technological collections.
Identification
For collectors the identification of a polymer type is important because it may provide useful information about the origin and authenticity of an object and also about how it is likely to deteriorate if not looked after carefully.
Although a few simple tests have been described which may allow the more common plastics to be identified (Braun 1996, van Oosten 1999, Shashoua 2008), the identification of plastics is not particularly easy. Some plastics such as cellulose nitrate have characteristic appearances (Figure 2) while others have distinctive odours. Cellulose acetate for instance, has a vinegar-like smell, whereas plasticised PVC has a ‘new car’ vinyl odour when warmed under running hot water. The odour of degrading or very new polymers is often informative.
Figure 2: 1930s cellulose nitrate imitation of tortoise shell powder bowl and lid (Copyright: Museum of Design in Plastics, Arts University, Bournemouth, Wallisdown, United Kingdom).
For some polymers it may be possible to use visual clues such as design, colour and trademarks to assist in identification. Function, touch, date of manufacture, flotation, solubility, density and flame tests may also allow a tentative identification to be made. Some of these tests are destructive however and should be undertaken with caution and only after careful familiarisation with the techniques involved. As the number and type of polymers is constantly growing, it is beyond the scope of this book to attempt to describe characteristic features of all commonly used plastics and rubbers. Readers are encouraged to refer to excellent texts such as Conservation of Plastics (Shashoua 2008) for further information.
Where a doubt exists as to the identity of a plastic it may be necessary to use analytical techniques such as Fourier transform infra-red (FTIR) spectroscopy, Raman spectroscopy or combined pyrolysis gas chromatography-mass spectrometry to allow a conclusive identification to be made. Shashoua (2008) provides copies of FTIR spectra of commonly collected polymers. Both FTIR and Raman spectroscopic analyses can be carried out non-destructively. Depending on the nature and amount of additional components present in an object the spectra can sometimes be difficult to interpret. Thus while these techniques will be able to identify at least the major organic component (the polymer) of a plastic, other techniques are more suited to the identification of plasticisers, stabilisers, dyes, pigments and fillers which may also be present in these substances (Shashoua 2008).
In addition to identifying the components of a polymeric substance, analytical techniques can also characterise the extent of degradation of the substance and often the degradation mechanism or mechanisms that are operating. This knowledge is very useful as it can be used to guide decisions regarding conservation and preservation options.
Deterioration
As plastics not only form part of collections but are often used to store and conserve artefacts, it is important to be aware of the agents that contribute to the deterioration of these materials. Although many polymers are considered to be imperishable and potentially a worldwide pollution problem, in reality they are not. Collectors on the other hand invariably wish that these materials were less prone to deterioration. Polymeric substances that are most prone to degradation include cellulose nitrate, cellulose acetate, polyurethane foams, PVC and rubber (Shashoua, 2008).
Degradation may be reflected by changes in the chemical composition and the physical and chemical properties of a polymeric object. These changes will affect the form and possibly the function of an object, therefore altering its significance and possibly its ability to be interpreted as part of a collection or a museum display.
Signs of deterioration may include:
- distinctive odours (e.g. camphor from degrading cellulose nitrate);
- changes in colour, shape or surface appearance (blooms, cracks or crazing);
- the corrosion of metal components; and
- the discolouration of associated packing materials
Deterioration of plastics may be due to chemical, physical or biological activity. As with most organic materials, polymeric objects will be affected by elements such as light (especially ultra violet radiation), heat, moisture, oxygen and pollutants. Unfortunately it is not easy to recognise deterioration in its very early stage (the ‘induction’ period). This is problematic because once started, deterioration can be autocatalytic, with the degradation products stimulating further attacks on their own polymer chains.
Light and heat can provide sufficient energy to break weak bonds in a polymer chain, leading to depolymerisation and subsequent alteration of its properties. As the rate of chemical reactions increases with increasing temperature, the rate and extent of degradation of polymers likewise increases as the temperature rises. As well as stimulating chemical change, heat can cause physical changes in thermoplastics, the distortion and buckling of vinyl records being a classic example.
In addition, oxidation reactions are enhanced by the presence of heat, light, metal ions and elevated relative humidity levels. Light and UV radiation in particular may cause colour changes via either alteration of the polymer itself or of the pigments and dyes present in the matrix. Discolouration of clear polymers is often an indication that oxidation has occurred. Low density polyethylene, polyamides (e.g. nylon 6) and acrylics (e.g. polymethyl methacrylate) all yellow when exposed to UV radiation and light, with the former two polymers also becoming more brittle on prolonged exposure.
Certain atmospheric pollutants, such as oxides of nitrogen and sulphur, are acidic and under conditions of high relative humidity can cause considerable damage to plastics. Polymers containing ester linkages such as cellulose acetate, polyesters and polyester-based polyurethanes are more prone to hydrolytic cleavage than are simpler molecules like polyethylene. Ozone is particularly damaging to rubber and to polymers containing unsaturated linkages in the polymer chains.
Inappropriate handling, cleaning or repairs can also accelerate deterioration due to the associated physical stresses and the impacts of some solvents. Areas of physical stress are more prone to chemical attack and bond-breaking. Solvents may not only dissolve a plastic but may also leach out plasticisers or cause the plastic to swell, thereby making the object more susceptible to chemical attack. These latter effects of solvent damage are often not immediately visible, with changes only becoming evident over a prolonged period.
Biological attack may occur, but this mode of deterioration is usually confined to semi-synthetic polymers derived from natural sources such as cellulose and casein and to some acrylic polymers (Shashoua 2008). This type of attack is favoured by high relative humidity levels.
Physical ageing is a less well known phenomenon in which polymeric materials (thermoplastics) continue to alter for long periods after their manufacture. Physical ageing is due to changes in the arrangement of the polymer chains with respect to one another. These changes are reflected in properties such as brittleness, stiffness, solubility and optical characteristics. A typical example is the slow hardening of natural rubber. The effects of physical ageing would be reversible were it not for the complications introduced by associated chemical degradation.
It is rare for only one mechanism of degradation to operate within a polymeric material. Usually changes in the chemical make-up of polymeric materials are reflected in corresponding changes in properties and appearance. For instance, evaporation or migration of plasticisers from a plastic will produce a material with reduced flexibility and increased brittleness. Shown below (Figure 3) is a cellulose acetate doll damaged by both the migration of plasticisers from the polymer and autocatalytic degradation caused by acetic acid released following hydrolysis of acetate groups in the polymer.
Figure 3: Cellulose acetate doll showing the effects of plasticiser loss and acetic acid attack.
Chemical attack on the polymer chain or on the cross-links between chains is likely to produce a weaker material with an altered resilience and changed stress relaxation. On the other hand, chemical changes which introduce cross-links into a plastic will produce a material with a reduced ability to stretch, reduced tear resistance and with an increased chance of suffering fatigue cracking. These sort of changes may be brought about by heat and oxidising conditions.
It is difficult to predict the precise behaviour of a particular object to a set of conditions. The formulations of many of the early polymers varied considerably, even for materials which were meant to be the same and the conditions of use and exposure of many polymeric objects will not be known. The use of impure materials in manufacturing processes also contributed to the variation in properties of early polymers. Thus with age, rubber may become hard, brittle and cracked or it may soften, becoming sticky and even liquefy. Plastics may shrink as they age and the surfaces of rubbers and plastics may craze or become chalky. More information regarding terms commonly used to describe polymer degradation, examples of these and summaries of the causes of degradation is provided elsewhere (Shashoua 2008).
It is likely that many recently produced polymers will have a greater resistance to deterioration due to the use of improved stabilisers and purer starting materials. Despite this, collection managers and conservators need to face the reality that some polymeric objects are inherently unstable and may simply have to be left to their inevitable fate.
Preventive Conservation
Rather than use the term preventive conservation when describing ways in which deterioration of polymeric substances can be slowed down, Shashoua (2008) prefers to use ‘inhibitive’ conservation. This is probably more accurate as it is generally accepted that once degradation has commenced in polymeric substances it cannot be stopped, merely ‘inhibited’ or slowed down. The type of preventive or inhibitive action that can be taken will be largely dependent on the following factors:
- the type of polymer;
- the current condition of the polymer; and
- the use to which the polymer is to be put.
Although there are no international guidelines regarding environmental conditions for polymers, knowing the polymer type and the likely causes of degradation will obviously assist in determining the optimum storage conditions. Precise specification of preservation guidelines is also complicated by the complex compositions of most polymers. Rarely are they just one component; rather they comprise a myriad of ingredients (co-polymers, fillers, flame retardants, plasticisers, dyes etc), many of which affect the stability and longevity of the object. Thus while some general guidelines may be given for polymeric substances, quite specific conditions must be maintained for those materials for which deterioration mechanisms are well known. Almost all polymers for example, benefit if light and UV radiation levels are limited and low temperatures are maintained. It is mainly polyester and ester-based polyurethanes however, that benefit from storage under low relative humidity conditions that limit hydrolytic degradation.
Environment
Conditions which slow the rate of deterioration of most plastics include:
- low temperatures;
- relative humidity levels of less than 50 per cent;
- low oxygen levels; and
- low light levels.
Storing most plastics and rubbers at low temperatures and low relative humidity (less than 50%) will retard the rate of chemical deterioration. Common wine refrigerators are quite good polymer storage units as they maintain low temperatures and relative humidity levels while allowing the condition of objects to be monitored visually. Note that nylon and casein formaldehyde polymers should be stored at about 60 % relative humidity to minimise shrinkage and brittleness.
As the rate of chemical reactions halve for every 10 °C drop in temperature, storage of most polymers in frost-free deep freezers or cold rooms will slow chemical deterioration. Caution is still needed however as some plastics may undergo stress cracking at very low temperatures and some materials such as painted animation cels (early cartoon materials) and laminates are at risk due to differential shrinkage rates of their components. In addition many polymers are brittle and therefore susceptible to physical damage if handled inappropriately while still very cold. Although research is continuing in this area, it is considered safe to store thin-walled polymers including cellulose nitrate, polystyrene, polyesters and acrylonitrile-butadiene-styrene copolymers in polythene bags in a freezer (Shashoua 2008). While thicker polymers can also be stored in freezers, they cannot simply be bagged and put directly into the freezer. To minimise the risk of damage, thicker polymers should be progressively cooled, moving from ambient conditions to cooler storage, then to a refrigerator before finally being moved into a freezer. This slow acclimatisation reduces the risk of water damage as the temperature is reduced. Conversely, thicker polymers should also be slowly acclimatised to warmer conditions should they be removed from a freezer (Shashoua 2014).
Self-indicating silica gel may be used to maintain a dry environment, but is not recommended for plasticised PVC as it has been shown to absorb phthalate plasticisers from the polymer, leading to accelerated deterioration (Shashoua 2001). As for all artefacts, it is wise to maintain stable conditions to minimise problems associated with adjustment to a different environment.
Oxygen exclusion will also enhance the longevity of most plastic and rubber artefacts. Achieve this by using an inert storage atmosphere (nitrogen or argon) or a chemical oxygen scavenger such as those described previously (see the chapter Mould and Insect Attack in Collections). There are obvious practical difficulties associated with building and maintaining a large, oxygen-free storage area. It is more practical to seal sensitive artefacts with oxygen scavengers in transparent, oxygen-impermeable envelopes. In 1991, British Museum staff sealed approximately 50 assorted polymeric objects in bags with oxygen scavengers. When these objects were examined about 10 years later, those objects still in sealed bags were essentially unchanged, unlike others that had been either inadvertently or deliberately opened (Dyer et al 2011). If this approach is adopted, monitor the activity of the oxygen scavengers and replace them when necessary.
Remember that placing polymers in a sealed container is not recommended for polymers like cellulose nitrate and cellulose acetates that produce damaging gases as they age and deteriorate. Enclosing these polymers in a sealed environment will accelerate their deterioration.
Transparency is an important feature of storage media as it allows objects to be monitored without having to open them. To adsorb degradation and other potentially damaging products in sealed containers, incorporate materials such as activated carbon, zeolites, potassium permanganate pellets, Artsorb® board, Microchamber™ papers and boards and buffered tissue paper in storage envelopes.
To minimise photochemical degradation, store objects in the dark or maintain light levels at 30 lux with a maximum UV exposure of 50 µwatts/lumen (1500 µW/m2).
Storage and Display
Regardless of the storage medium it is essential to regularly monitor the condition of plastic artefacts, especially when they are stored in closed environments. In these situations, if degradation continues, deterioration may be accelerated by the build-up of a harmful microenvironment in the closed system. The deterioration of cellulose acetate for example is usually accompanied by the release of acetic acid vapours. These would build up in a closed system and increase the rate of degradation of an already degrading object. Acid detection (A-D) strips, which change colour as the acidity of an environment changes, can be used in a closed system to detect the build-up of acid vapours. They can also be used to determine when a zeolite or activated charcoal absorbing material has lost its activity and needs refreshing. Consult a conservator to receive the most recent advice on the use of these and other monitoring methods.
Isolate deteriorating plastics from other materials to prevent damage to unaffected objects. Do not store deteriorating objects in a closed environment; instead, store them in open metal cupboards, protected from dust and light by muslin (or similar) curtains or in ventilated polypropylene containers. This arrangement will permit maximum ventilation. Use buffered acid-free paper to scavenge free acids and acid-free card to line the metal shelving.
Acid-free paper, silicone release paper, polyester wadding and polyethylene foams are suitable for wrapping or supporting most polymers.
Make replicas of particularly valuable objects and use these for display. Store the originals under the best conditions possible (cold, dark and with both low relative humidity and oxygen levels).
Support flexible objects, such as rubber-based materials, at all times. As rubber degrades and plasticisers are lost from some plastic objects, artefacts made from these materials may harden over time. Support these objects in their correct shape using polyethylene foam or tightly crumpled acid-free tissue paper.
Do not allow objects which contain a high proportion of plasticisers, such as those made from cellulose acetates and PVC, to come into contact with each other or with other plastics. This will avoid the possibility of migration of plasticiser from one object to another, a process which could lead to either the softening of objects or of their ‘welding’ together.
Special mention needs to be made of objects composed of cellulose nitrate, cellulose acetate, plasticised polyvinyl chloride, polyurethane foams and rubber as these polymers are particularly susceptible to deterioration.
Cellulose nitrate-based materials were produced extensively during the period 1860 to 1930 and include imitation tortoise shell and ivory objects, cutlery handles, toys, films and negatives. As cellulose nitrate objects degrade there is an increased risk of fire. In addition, the release of acidic or oxidising gases by degrading cellulose nitrate objects increases the risk of continued damage to the object itself and damage occurring to metal and other objects that may be stored in close proximity. Camphor was used as a plasticiser in some cellulose nitrate objects. Loss of the volatile camphor from the polymer is usually indicated by a characteristic smell, increased brittleness and cracking of the object’s surface (Figure 4).
Figure 4: Cracking in a cellulose nitrate buckle and accelerated corrosion of the attached metal bar due to nitric acid release from the degrading plastic (copyright Cordelia Rogerson, www.materials.ac.uk/events/doc/plastics-rogerson.ppt).
Segregate cellulose nitrate objects from other objects in a collection in a well ventilated, fire-proof area. Do not circulate vented air to other areas of the collection. Obviously isolation from heat and ignition sources is also essential.
Store cellulose nitrate motion picture film in perforated cans to allow gases to vent and store negatives in buffered paper envelopes. If stored in boxes, add activated charcoal cloth, charcoal paper, Microchamber™ papers and boards or other zeolites to absorb damaging gases that are given off as the polymer degrades.
Copy degrading films and negatives to ensure that the information that they contain is not lost. As specialist equipment is needed to reformat these materials, consult a conservator.
Careful monitoring is critical in these cases. Stages of degradation include progressive yellowing, the formation of bubbles or foam, embrittlement and shrinkage. Store these artefacts at 2 – 5 °C, at a relative humidity between 20 and 30 % and at maximum light and UV levels of 50 lux and 75 microwatts/lumen respectively (Shashoua 2008). Dark storage is strongly recommended.
As the gases given off by degrading cellulose nitrate objects are damaging to health, take appropriate precautions when working with these materials. Work in a well ventilated area and wear protective clothing including nitrile gloves.
Cellulose acetate objects including films, combs, toys and other moulded goods were mainly produced from the late 1920s to the 1970s. Whereas films and fibres were made primarily from cellulose triacetate, three-dimensional objects were usually made from cellulose diacetate.
Many cellulose acetate objects contain plasticisers (often phthalates) which can migrate from the polymer structure and acetate groups that can react to produce acetic acid. Migration of plasticisers to the polymer surface can produce an acidic environment and warping of the plastic whereas hydrolysis of the acetate groups produces acetic acid and a characteristic vinegar-like smell. If cellulose acetate butyrate is present in the polymer, it will smell more like rancid butter as it degrades. The presence of bubbles or crystals (plasticiser) on the surface of an object or a mild vinegary smell is an indication that active degradation has commenced.
Acetic acid will break down the polymer chain and can also attack any susceptible objects like metals, textiles and paper that are in the same vicinity. It is important therefore to segregate cellulose acetate objects from other sensitive objects.
As well as storing cellulose acetate films and negatives in appropriate conditions, copy them so that their original information is preserved. If there is no option but to store cellulose acetate films in sealed metal or plastic cans, incorporate zeolite packages in the containers to absorb damaging moisture and acetic acid vapours (Shashoua 2008).
Store cellulose acetate objects in a ventilated area, at 2 – 5 °C, at a relative humidity between 20 and 30 % and at maximum light and UV levels of 50 lux and 75 microwatts/lumen respectively (Shashoua 2008). Again dark storage is also strongly recommended.
Plasticised polyvinyl chloride objects, including toys, waterproof clothing and electrical cabling were initially produced in the 1940s and are still made today. Pure PVC materials are hard and rigid, with plasticisers added to give them flexibility and softness.
Discolouration (yellowing and darkening) and warping are often the first signs of degradation. Other signs include cracking and an acidic and/or ‘plastic’ smell from the breakdown of the polyvinyl chloride or migration and loss of the phthalate plasticisers respectively. As a PVC object degrades, hydrochloric acid may be given off. This may be reflected in the corrosion of metal components of an object or brittleness of paper wrappings, signs that degradation has started.
The migration of plasticisers is a major problem as the PVC objects lose flexibility, shape and integrity and related degradation is accelerated. Phthalate plasticisers can form a ‘bloom’ on the surface of objects. This should not be removed unless absolutely necessary as it will merely encourage the migration of more plasticiser to the surface. Isolate objects from direct contact with other objects but exercise caution if wrapping is the only isolation alternative as there is a risk of damage to soft, deteriorating surfaces.
Store plasticised PVC objects in cool, dark conditions, enclosed in non-absorbent materials like glass and polyester envelopes. The use of non-absorbent materials minimises plasticiser loss from the polymer. Low density polyethylene is not a good storage medium because it readily absorbs plasticisers (Shashoua 2008). Ideally these objects should also be stored in an oxygen-free environment.
It may be necessary to support some PVC objects in their preferred shape as they age and become more brittle.
Polyurethane foams have been used in the production of toys, packaging, textiles (including fake leather) and cushioning from the 1940s to the present.
Polyether-based polyurethanes are susceptible to oxidative degradation, particularly in the presence of light, while polyester-based polyurethanes, although more resistant to oxidation, are more vulnerable to hydrolysis in moist conditions. Degradation is usually indicated by a pungent odour, discolouration and/or loss of mechanical properties. Degradation can be quite rapid because of the large surface area of these foams and can result in the complete crumbling of the whole object.
If possible separate deteriorating foam objects from others and unless opting for low oxygen storage, provide good ventilation.
Store polyurethane foam objects in cool, dark, low oxygen conditions and polyester-based polyurethanes in low relative humidity conditions (20 – 30 %, see the chapter Preventive Conservation: Agents of Decay). The addition of activated charcoal or zeolites to any sealed storage system will help to minimise the build up of potentially damaging degradation products.
Synthetic rubber products have been available from the late 1830s when sulphur was introduced to cross-link the natural rubber chains. The hardness and elasticity of the products was determined by the amount of incorporated sulphur, with the hard rubbers like ebonite and vulcanite containing up to 30 % sulphur. As rubbers were also prone to oxidation, stabilisers were often added to rubber products. The off-gassing of sulphur-based vapours and the migration of volatile stabilisers are major problems of degrading rubber.
Because of the different initial properties of assorted rubber objects, their deterioration may be indicated in number of different ways. They may discolour, become harder or softer, less elastic, more brittle, stickier or even develop a more powdery surface. Hydrogen sulphide gases are emitted and sulphuric acid may be deposited on the surfaces of degrading objects. Paper or plastics in contact with rubber objects may become stained as coloured stabilisers migrate from the rubber. Silver is particularly susceptible to tarnishing in the presence of rubber-based objects.
As rubber objects emit damaging gases, separate them from other objects, preferably in a well-ventilated area. If ventilation is not possible, seal them in enclosures with activated charcoal or zeolites.
As oxidation is the major degradation pathway for rubber objects, store them at low relative humidity levels, low temperatures and in low oxygen environments. Although low temperatures will result in an alteration of the crystallinity of the rubber, causing it to lose its elasticity, this change is reversible on warming (Smithsonian Institute). Low temperature storage is acceptable for rubber objects in either good or degraded condition. If rubber objects are sealed in low oxygen environments, make sure that acid scavengers (such as activated charcoal or zeolites) are also included with the oxygen scavengers to avoid the build-up of damaging microenvironments.
Regular monitoring of the condition of objects is essential.
Treatments
Interventive treatments should only be considered if they are absolutely necessary to minimise further deterioration or to preserve the significance of an object. Approach the treatment of plastic materials with utmost caution. Before undertaking any treatment, determine the type of plastic in the object. Because of the sensitivity of many plastics to solvents, cleaning agents and adhesives and the difficulty involved in identifying them, contact a conservation professional for advice rather than attempting to treat objects yourself. There is a significant risk of damage to objects otherwise.
Cleaning
Reasons for cleaning plastic objects include the removal of surface degradation products, the improvement of unsightly appearances and the removal of dust which can enhance degradation. Few reports have been made about cleaning and stabilising degraded plastic and rubber because of their delicate nature, susceptibility to further damage and relative impermeability.
Physical cleaning may involve either brushing, gentle wiping with soft, lint-free and/or microfibre cloths, vacuuming or blowing air to remove adherent particles. Great care must be taken if physical methods are used because of the risk of damage to the plastic surface.
Although aqueous cleaning may be problematic for polymers that are susceptible to hydrolytic breakdown (such as semi-synthetic polymers, polyesters and polyester-based polyurethanes), some plastics may be safely washed with warm water containing small amounts (3 - 5 %) of a non-ionic detergent (for example, Lissapol, Teric N9, Arkopal N090) and a soft cloth or brush. Objects should never be immersed in water however. To avoid the possibility of swelling dry washed objects immediately after wet cleaning with either a soft cloth or tissue. This approach has been successfully used on plasticised PVC (Huys and van Oosten 2005) and on a polyether-based polyurethane sculpture (Winkelmeyer 2002). It is most important to use soft, lint-free swabs that minimise surface abrasion when applying the detergent and during subsequent rinsing and drying.
Do not use water on (Morgan 1992):
- vulcanite (hard rubber) that is beginning to degrade;
- surface-dyed casein;
- degrading cellulose nitrate or cellulose acetate where the surface is cracked or crazed; and
- objects which contain metal components which may corrode.
The use of non-aqueous solvents is not generally recommended due to the increased possibility of damage occurring to the polymer (Shashoua 2008). If it is necessary to use a non-aqueous solvent either as a cleaner, to remove an adhesive or to reverse an earlier repair for instance, it is best to consult Hildebrand solubility parameters (as modified by Hansen 2007) to ensure that the solvent used will not damage the polymer. Once a potential solvent has been selected, test it on an inconspicuous part of the object before use because the solubility properties of the polymer may have changed as it degraded.
Some collectors have used commercial acne cleaners to remove green copper staining from doll’s PVC skin and commercial polishes to remove scratches to improve the appearance of plastic objects (Shashoua 2008). As most commercial polishes are silicone-based, the latter treatment is likely to be irreversible. In addition these treatments have not been evaluated to determine any possible long-term effect on polymer preservation.
Stabilising Deteriorating Objects
There have only been a few descriptions of attempts to stabilise deteriorating polymeric objects.
Despite the reservations expressed above (Morgan 1992), deteriorating cellulose nitrate beads were washed in water to remove acid and then soaked in very dilute sodium carbonate solution to neutralise any remaining acid and to buffer future acid formation. This approach was successful in stabilising these objects. It would be unlikely to be effective in the treatment of large objects due to the lack of porosity of cellulose nitrate (Green and Bradley, 1988).
The consolidation and repair of a pair of severely degraded rubber bathing shoes has also been described (Maltby 1988). The shoes had areas of differing degradation, soft and slightly tacky in some areas, hard and brittle in other areas and supple and undegraded elsewhere. Specialised techniques were used to reshape the shoes, fill lost areas and strengthen fragile areas. A fitted padded support was constructed from ethafoam and a specially designed display mount was prepared. As the shoes were extremely fragile and could not be strapped in place, a tin plate insole was inserted in each shoe and magnets fixed under the acid-free mount. Removal of the magnets allowed the shoes to be ‘freed’. To enhance the longevity of the shoes they were stored in a nitrogen atmosphere.
Repairs and In-Painting
Before embarking on the repair or treatment of plastics there is a number of factors that must be considered. These include the:
- basic nature of the plastic including the extent of degradation and surface tension;
- solvent and plastic interaction;
- properties of possible adhesives including coefficients of expansion, flexibility, ageing characteristics and refractive indices; and
- necessity for pre-treatments to improve adhesion
To repair or even in-paint a plastic object effectively, it is necessary for a bond to form between the applied adhesive or in-painting medium and the substrate. If this is not the case then there will be no effect as the adhesive will not hold and the paint will fall off. Implicit in this process is the use of a solvent which will not adversely affect the plastic in some way.
While water-based adhesives would seem to be the least likely to damage most polymers, large differences between the surface tensions of these materials limits the effectiveness of these adhesives. Although information is available which matches adhesive types with specific plastics and descriptions are given of other joining techniques including heat and solvent-welding, it is strongly recommended that a conservator is contacted before attempting this type of treatment (Blank 1988, Shashoua 2008).
Consolidating, impregnating and filling plastics are highly specialised tasks. While successful treatments have been applied to crumbling, cracking polyurethane foams and crazing cellulose nitrate objects, these types of treatments require specialist knowledge (Shashoua 2008).
Labelling Objects
If labelling of plastics is needed, use either a soft pencil on a discrete surface (suitable for polyethylene and PVC) or an ink based on an acrylic dispersion medium with carbon black or rutile titanium white as pigments (Shashoua 2008). While tie-on labels with cotton tape can be safely used, there is always a risk of detachment or loss of the label. Avoid applying a barrier layer between the plastic and the ink and also avoid the use of solvent-based inks and paints, rubber bands or adhesive tapes.
Summary
- Store most plastic and rubber materials at low temperatures (a freezer is acceptable for most polymers) and maintain relative humidity levels below 50%.
- Maintain low oxygen environments and low light levels (30 lux with a maximum UV exposure of 1500 µW/m2, but preferably no light) to enhance stability of plastics.
- Monitor the condition of plastics carefully to prevent damage to nearby artefacts.
- Support flexible objects in their desired shapes.
Bibliography
Blank, S., 1988, Practical answers to plastic problems, in Modern Organic Materials, Preprints of the Meeting, Edinburgh, 1988, Scottish Society for Conservation & Restoration, Edinburgh, pp. 115-122.
Braun, M.D., 1996, Simple Methods for the Identification of Plastics, 3rd edition, Hanser/Gardner Publishing Inc., Cincinnati.
Dyer, J., Ward, C., Rode, N., Hacke, M. and Shashoua, Y., 2011, Reassessment of Anoxic Storage of Ethnographic Rubber Objects, in Preprints of 16th Triennial Conference, ICOM-CC, Lisbon, Portugal, 19–23 September 2011, J. Bridgland (Ed.), pp. 1-10.
Green, L. and Bradley, S., 1988, An investigation into the deterioration and stabilisation of nitrocellulose in museum collections, in Modern Organic Materials, Preprints of the Meeting, Edinburgh, 1988, Scottish Society for Conservation & Restoration, Edinburgh, pp. 81-96.
Hansen, C.M., (Ed.), 2007, Hansen Solubility Parameters: A User’s Handbook, 2nd Edition, CRC Press, New York.
Huys, F. and van Oosten, T.B., 2005, The ‘Aeromodeller 00-PL; the conservation of a PVC balloon,, in Preprints of the 14th ICOM-CC Triennial Meeting, The Hague, 12 -16 September 2005, James & James Ltd, pp. 335-342.
Kaufman, M., 1963, The First Century of Plastics - Celluloid and its Sequel, Plastics and Rubber Institute, Chameleon Press, London.
Kroschwitz, J.I., (Ed.), 1987, The history of polymer science, in Encyclopedia of Polymer Science and Engineering, vol. 7, Wiley-Interscience, New York.
Lavédrine, B., Fournier, A. and Martin, G., (Eds), 2012, Preservation of Plastic Artefacts in Museum Collections, Paris: Comité des travaux historiques et scientifiques.
Maltby, S.L., 1988, Rubber: the problem that becomes a solution, in Modern Organic Materials, Preprints of the Meeting, Edinburgh, 1988, Scottish Society for the Conservation & Restoration, Edinburgh, pp. 151-157.
Morgan, J., 1992, The cleaning and care of plastic artifacts, Polymer Preprints, vol. 33, pp. 643-644.
Mossman, S.T.I., 1997, Early plastics: Perspectives 1850-1950, Cassel, London.
Shashoua, Y.R., 2001, Inhibiting the degradation of plasticised poly(vinyl chloride) – a museum perspective, Ph.D. thesis, Danish Polymer Centre, Technical University of Denmark.
Shashoua, Y., 2008, Conservation of Plastics: Materials Science, Degradation and Preservation, Butterworth-Heinemann, Oxford, United Kingdom.
Shashoua, y., 2014, A safe place: Storage strategies for plastics, in Conservation Perspectives, the GCI Newsletter, Conservation of Plastics, vol 29 (1), pp. 13–15.
Smithsonian Museum Conservation Institute, http://www.si.edu/mci/
Van Oosten, T.B., 1999, Identifying plastics: Analytical methods, in A. Quye and C. Williamson (eds), Plastics: Collecting and Conserving, NMS Publishing Limited, Edinburgh.
Winkelmeyer, I., 2002, Perfection for an instant-restoration of a polyurethane soft sculpture by John Chamberlain, in T. van Oosten, Y. Shashoua and F. Waentig (eds), Postprints of the ICOM-CC Modern Materials Interim Meeting, 12 – 14 March 2001, Siegls Fachbuch Handling, pp. 153-164.