Glass
C. Corvaia
Introduction
Glass manufacture is believed to have originated around 5000 years ago in Mesopotamia or Egypt. The glass beads, figurines and vessels produced were similar in appearance to ceramic objects. They were made using the techniques of core forming, mosaic, fusion, mould casting and cold cutting. Glass blowing was developed approximately 2000 years ago, most probably in Syria. The techniques for hand working glass have continued essentially unchanged since that time.
Ancient glass was formed from a mixture of silica (sand, crushed quartz or flint), alkali (soda or potash from plant ash) and lime (probably included as an impurity in the sand). Oxides of metals such as copper, iron, cobalt and manganese were added to produce coloured glass. Some glasses which were not purposely coloured had yellow or green tinges. This was due to iron oxide impurities in the sand. A colourless glass was first produced about 1900 years ago by adding manganese dioxide to the usual glass components.
Until about 1000 years ago soda from the ashes of marine plants were universally used to modify the silica network. After this time the glass makers of Northern Europe replaced soda with potash obtained from beech wood ash. The resultant glass was known as ‘forest glass’ and was mainly green or brown in colour.
By the 15th century Venice was established as the most prominent glass manufacturing centre in Europe. ‘Cristallo’, a fine clear glass was developed by adding lime to the soda-silica mixture. In Bohemia in the early 16th century, a clear solid crystal glass was produced when lime was added to the potash-silica mixture. This glass was ideal for cutting and engraving. Lead glass, also known as ‘glass of flint’, was developed in Britain in the 17th century. This glass, with a 30 per cent lead oxide content, is characterised by its clarity and brightness.
Mould casting was revived in North America in the early 19th century. The use of press moulding allowed intricate patterns to be produced cheaply and quickly. Early pressed glass objects were made predominantly from colourless lead glass. In 1864 a ‘lime’ glass was developed which was most suitable for pressing. It resembled lead glass but cost less. This was important as the production of glass containers became mechanised in the late 19th century. Hand blown bottles were still produced as late as the 1930s but only for certain types such as pharmaceutical bottles and cosmetic ware.
Most of the glassware used during the 18th and 19th centuries in Australia and other parts of the then British Empire was imported from Britain. In comparison with the common ‘black’ glass bottles, there is a scarcity of the colourless and pale green flint glass objects on early Australian sites. This is due to manufacturing limitations and differences in excise duty that existed between flint glass and black glass in Britain from 1746 to 1845 (Boow 1991). Older, cheaper methods of manufacture, such as those using wooden moulds and push-ups, were continued for black glass bottles until about 1870.
Deterioration
Apart from the obvious fragility of glass and its tendency to crack and break, there are other forms of deterioration which occur when glass is in contact with moisture and aqueous solutions (Figure 1).
Figure 1: Examples of glass deterioration in a bottle collection
Surface weathering produces effects which vary greatly, from a loss of transparency to the formation of iridescent layers, crizzling and scaly encrustations.
The reaction of glass with water can be described as “an inward diffusion of water molecules, which then react with non-bridging oxygen atoms to produce hydroxyl ions that migrate out with the alkali cations” (Newton 1989). The surface layer of the glass becomes alkali depleted and protons (hydrogen ions) replace the alkali ions in the glass network. As protons are smaller, this results in shrinkage of the leached surface layer of glass. When the glass dries various forms of deterioration may be observed. The surface may become opaque or iridescent and fine lamellae or thick flakes may fall away.
When flat sheets of glass are stored ‘face to face’ in a humid environment, the trapped moisture will quickly form a leaching solution and attack the glass.
Crizzling, the formation of very fine surface cracking, occurs in glass with a disproportionate composition (excess alkali and insufficient lime). This type of composition is often found in glass of the 16th to the 18th centuries. Crizzled glass objects ‘weep’ when placed in a humid environment, with the droplets of moisture on the surface continuously leaching the excess alkali.
Other environmental influences which adversely affect glass include:
- carbon dioxide and sulphur dioxide which combine with moisture in the air to produce weathering crusts of calcite (CaCO3) and gypsum (CaSO4) as found for example, on old window glass;
- slightly acidic or alkaline soils;
- soluble salts which are inevitably present in glass recovered from a marine environment. If the glass is allowed to dry before being thoroughly washed, crystallising salts will cause surface exfoliation; and
- exposure to sunlight. Decolourised glass, which contains manganese salts, develops a purple hue after prolonged exposure. This effect has even been found in shards recovered from shallow water sites.
Preventive Conservation
Environment
Optimum conditions for storage and display of stable glass objects are 40 - 60% relative humidity and a temperature range of 15 – 25 °C with maximum variations of 5 % and 4 °C respectively within any 24 hour period.
Relative humidity control is important as a moist environment will adversely affect any glass surface and a desiccating environment will cause further damage to weathered glass. In addition, adhesives used for repairs may ‘cold flow’ if exposed to high relative humidity or high temperatures.
High temperatures may damage painted, crizzled or weathered glass. After appropriate treatment, keep chemically unstable, crizzled and weeping glass at 42% relative humidity. This strict condition is necessary as higher relative humidity levels will dissolve salts and lead to their migration while lower relative humidity levels increase the risk of cracking. Use a sealed container and conditioned silica gel or an appropriate saturated salt solution to maintain this relative humidity level and seek advice from a conservator.
Light levels are not critical for most glass as fading is not usually a problem. Sunlight and other UV sources will affect decolourised glass, giving it a purple hue. Light levels of up to 300 lux, with a UV content of 75 µwatts/lumen (22,500 µwatts/m2) are acceptable for stable glass objects.
It is important to avoid heat build-up from lighting. Place display lighting outside showcases and if possible direct light onto glass objects by reflection.
Handling
Glass is a fragile material which is easily broken if not handled carefully. Take the following guidelines into account when handling glass objects:
- check for breakages or failing adhesive;
- remove any loose parts such as lids or stoppers before carrying;
- only carry one object at a time with the weight supported uniformly and one hand under the base;
- do not wear cotton gloves as glass surfaces are smooth and slippery. Instead handle objects with bare, clean hands or disposable rubber gloves;
- line trays used to carry objects with bubble wrap or cottonwool covered in tissue paper. Insert bubble wrap or crumpled tissue paper padding between objects; and
- keep handling of iridescent or weathered glass to a minimum.
Storage and Display
Do not store flat glass ‘face to face’ as trapped moisture will damage the glass surface. Use perforated acid-free board to separate the glass sheets and allow air exchange.
Store glass vessels in rigid cardboard boxes padded with inert polyethylene foam, bubble wrap or crumpled acid-free tissue paper. Use foam core board or ethafoam to build special cradles for top heavy or damaged objects.
Unless the surfaces are properly sealed (see the chapter Preventive Conservation: Agents of Decay), do not use wooden cupboards or showcases for storing or displaying glass objects. Acid vapours given off by hardwoods such as oak and birch and by composite boards are damaging to glass that has a high soda content. Enamelled or powder-coated metal cabinets or shelving are recommended. Line shelves with a non-slip material such as closed cell polyethylene foam. Attach this foam firmly to the shelving.
Treatments
Examination
Thoroughly examine glass objects before storing, displaying or treating them in any way. Photographs, as well as notes and drawings, are useful for documenting the condition of objects before and after treatment.
Identify and clearly describe signs of deterioration. These include the presence of:
- cracks;
- old repairs;
- missing parts; and
- surface deterioration such as opacity, iridescence (or opalescence), crizzling, pitting, delamination and encrustations.
Also note any marks that indicate the place, date or method of manufacture and original contents of bottles and jars. Record the presence of any content residues. Ensure these residues are maintained during any subsequent treatment.
Keep accurate records of any treatments that are carried out and any changes that occur to the object while it is in storage or on display.
Cleaning
Only clean glass if it is in sound condition. Do not brush or wet any glass that has poorly adhering paint or surface weathering. Do not clean or chemically treat degraded glass (for example, exfoliated, crizzled, weeping, encrusted) or painted glass without consulting a conservator.
Guidelines for cleaning sound glassware are described below:
- use dry methods of cleaning first;
- remove surface dirt with a soft, clean cloth or a brush and gentle vacuum suction;
- if wet cleaning is necessary, then examine the object for any previous restoration, as some adhesives may be affected by water;
- clean the surface of repaired objects by applying distilled water with cotton swabs;
- to wash sound glassware, use cold or warm water (one litre) and add either a few drops of a concentrated, non-ionic detergent (such as Lissapol) or about 100 ml of pure soap solution. (The soap solution is prepared by dissolving one gram or about one teaspoon of pure soap flakes in a little hot water and adding enough cold water to make one litre.);
- use a large plastic container or a sink lined with towels;
- do not leave glass to soak in the water;
- only wash one object at a time;
- use soft brushes, cotton swabs or pliable sticks to remove stubborn dirt from wet glass;
- thoroughly rinse the glass with distilled or deionised water (in a large container) and drain on cloth or paper towelling;
- dry glass immediately with a soft cloth or by immersing it in a bath of methylated spirits; and
- use methylated spirits to remove stubborn dirt. Apply with a cotton swab, after testing any painted areas.
Rinse ‘weeping’ glass gently in deionised or distilled water. Do not leave it to soak. Dry it quickly and thoroughly by passing through two baths of methylated spirits. The ideal storage conditions for this type of glass is in an environment maintained at a relative humidity of 42 %. This will avoid further ‘weeping’.
Desalination
Place glass recovered from waterlogged land sites or marine environments immediately into sealed polythene bags to prevent drying. For glass recovered from the sea, partially fill the bags with seawater.
Washing is necessary to remove soluble salts, especially chloride salts, which will otherwise damage the glass when it is dried. Guidelines for desalination are described elsewhere (see the chapter Ceramics).
Removing Encrustations
Calcium encrustations can be removed mechanically from sound glass using pliable wooden spatulas and brushes while the glass is being desalinated. Calcium encrustations harden and become more difficult to remove if allowed to dry. Remove surface encrustations with great care as some forms of surface degradation, such as iridescence and exfoliation, are not apparent when the glass is wet.
Using polyphosphate water softeners, such as ‘Calgon’, to remove calcium encrustations is not recommended for any weathered glass as these are damaging to the glass (Horie 1989).
Use water softeners with caution for the treatment of strong, unweathered glass. A 2 % solution of Calgon in cold or warm water, sufficient to cover the object, may be used for this purpose. Initially the glass may be soaked for a few hours, periodically brushing away the calcium encrustations as they soften. Monitor this treatment carefully.
Repair
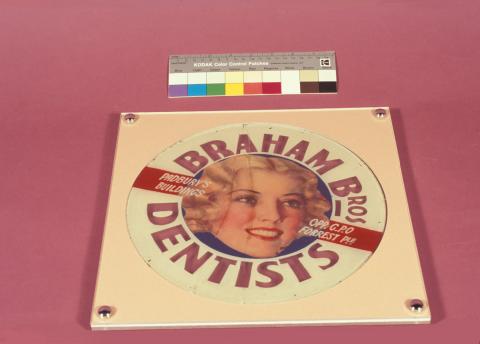
Joining broken glass is made difficult by the transparency of the material, its smoothness and the lack of ‘tooth’ or ‘key’ on broken edges (Figure 2).
Although epoxy resins have been used to repair glass their general use is not recommended. They tend to yellow as they age and are difficult to remove. A few epoxy resins have been developed specifically for conservation use and these have good ageing properties. They are available from conservation materials suppliers but appear to have a limited shelf life and are very expensive.
Some acrylic and polyvinyl acetate adhesives used for ceramic repair, Paraloid B-72 and UHU All Purpose Adhesive respectively, are also suitable for glass repair. The principles, guidelines and techniques for repair and reconstruction previously outlined for ceramics apply equally for glass fragments (see the chapter Ceramics). Points specific to glass are described below:
- before joining, degrease the edges of glass fragments with a cotton swab soaked with either methylated spirits or acetone;
- do not place tape, used to hold fragments together, on fragile surfaces;
- if the artefact is flat, lay fragments together on a flat surface and hold with tape;
- place a release material, such as wax paper or silicone release paper, under the glass fragments to prevent excess adhesive adhering to the support;
- paint the adhesive lightly over the joins. The adhesive will be drawn into the joins by capillary action; and
- remove excess adhesive later, using a sharp scalpel.
Figure 2: Broken glass – advertising sign.
(a) The glass sign before conservation and restoration.
(b) The glass sign after conservation and mounting behind Perspex.
Often synthetic casting resins are used for filling losses in glass. Gap filling causes some stress to the glass and the resins have a tendency to yellow. Only carry out filling if it is structurally or aesthetically necessary. It is a complicated procedure and consultation with a conservator is essential.
To strengthen a crack in glass to avoid further damage, dilute adhesive with an appropriate solvent and paint along the crack. Even a well repaired join in clear glass usually remains visible. This occurs because the refractive indices of the glass and that of the adhesive are usually different.
A broken glass stem may require dowelling as well as adhesive for a successful repair. The difficulty of drilling precise holes into a broken glass stem can be overcome by using a metal collar and a glass drill bit (Jackson 1982). The dowelling technique is complex and it is best to seek advice from a conservator.
Consolidation of glass is not generally recommended. If it is considered necessary, consult a conservator. Exfoliating, iridescent glass will lose its ‘rainbow’ colouring if a consolidant is used.
Bottles
Bottles and jars form the basis of many collections. As most bottles are made from glass the aspects of deterioration, care and conservation outlined earlier in this chapter apply to these objects. It is most important to be aware of the history of collected bottles, as the burial environment from which they were recovered will influence deterioration processes.
Deterioration
In addition to the usual factors which affect the deterioration of glass, internal stresses will be present in hand- or machine-blown glassware. This is due to differences in cooling and shrinkage rates between the thin and thick sections of the molten glass vessel. Exposing bottles to sudden changes in temperature may upset the balance of these internal stresses, resulting in the glass fracturing. For this reason always allow glass to adjust gradually to new environmental conditions. Such allowances should be made following excavation, washing and movement from one climatic region to another.
Weathered glass attains a variety of colours and textures depending on the environmental conditions to which it has been exposed. Bottles that have been in a moist environment will become iridescent or silvery and the surface may exfoliate. Glass buried in an alkaline environment such as found in guano sites for example is characterised by pitted, opaque surfaces.
Clear glass exposed to sunlight for a long time may become purple, indicating that manganese was used in its manufacture. Some clear glass will become amber, an indication that selenium rather than manganese was used as the decolourising agent. As selenium was used between 1915 and 1930 and manganese was used predominantly between 1880 and 1915, these forms of deterioration are important as dating indicators.
As long as they are placed in a suitable environment, most weathered glass objects will not deteriorate further after soluble salts have been removed from them.
Preventive Conservation
During and after excavation, do not allow wet bottles to dry. Keep plastic containers with tight-fitting lids, polyethylene bags and bubble wrap on hand to store the glass as it is recovered.
Store and display glass bottles under the conditions previously recommended for glass objects. In view of the internal stresses present in bottles, control temperature and relative humidity conditions carefully.
Treatments
Cleaning
Before washing, allow bottles excavated from wet sites to stand for 24 hours in a partially closed bucket with 10 millimetres of water covering the base. This will allow the bottle to adjust to the ambient conditions.
Gradually humidify dried bottles that require washing in a similar fashion.
Remove softened accretions with a nylon brush, such as a toothbrush. Loosen any sand packed inside bottles with a pointed wooden tool and then gently shake it out. Avoid using metal probes as these may scratch and mark the glass. Water will also help loosen and remove packed material from inside bottles.
Soften stubborn accretions and remove soluble salts by immersing the bottles in tap water. Soaking for a few days may be necessary. Do not use warm or hot water as the temperature change may crack the bottles.
Chemicals are generally not recommended for cleaning glass bottles. Although a quick result may be achieved with the use of acids, soda or household bleach, these chemicals will attack weathered glass and leave harmful residues.
An alternative cleaning technique involves the use of an ultrasonic bath. A high frequency sound produces shock waves, which loosen dirt from the glass object. Use this technique cautiously however, as friable glass may disintegrate in the process.
Corks, Caps and Labels
Some bottles may retain the original contents, cork, lead cap or paper label. In order to keep as much information about the bottle as possible, conserve these objects intact.
Paper labels are very fragile and may be obscured by dirt and mould. Remove dirt from the glass and paper by gentle dry brushing. Take precautions so that dust which contains mould spores is not inhaled. A cotton bud moistened with water and wooden points can be used to clean the glass. Do not wet the paper during the cleaning process.
Lead caps on bottles are usually corroded and require chemical treatment to stabilise them. Although details of lead treatment are given elsewhere (see the chapter Metals) consult a conservator before embarking on any treatment of lead caps.
Following all treatments, microcrystalline wax paste can be applied to the lead and any exposed cork to seal the bottle and contents. A suitable wax paste and its method of application are described below.
| microcrystalline wax | 5 g |
| white spirit | 10 ml |
- apply the paste with a small, stiff brush and allow it to dry; and
- repeat the process until a layer approximately 2 mm thick coats the lead and exposed cork.
If no lead cap is present but the cork is sound and the contents have been retained, the bottle may be cleaned and washed as described in the section above for empty glass bottles.
After cleaning, seal the bottle to ensure the cork remains moist and swollen and that the contents do not evaporate. Apply microcrystalline wax paste to the cork and lip of the bottle (as described above).
Consult a conservator if more information on sealing bottles is needed.
Polishing Glass
Do not use abrasive powders or chemicals to polish glass. In these processes a layer of the original object is destroyed, breakage may result from heat build-up and chemical residues may be deposited which may cause further damage.
Summary
- Store sound glass in a stable environment with temperature and relative humidity ranges of 15 - 25 °C and 40 – 60 % respectively with maximum variations of 4 °C and 5 % respectively in any 24 hour period.
- Handle glass objects with disposable rubber gloves or clean bare hands.
- Carry objects one at a time, with support at the base and side. Alternatively use a padded tray to move a group of objects.
- Never store flat glass face-to-face. Separate the sheets to allow for air exchange.
- Examine objects thoroughly and record their condition, old repairs, makers’ marks or content residues before undertaking any cleaning or other treatment.
- Consult a conservator before treating degraded or painted glass.
- Desalinate glass recovered from burial or marine sites. Keep the glass wet following excavation and during desalination.
Bibliography
Boow, J., 1991, Early Australian Commercial Glass: Manufacturing Processes, Ed. J. Byrnes, Heritage Council of NSW, Sydney.
Craft, M., 1992, Decorative arts, in Caring for Your Collections, National Committee to Save America’s Cultural Collections, Arthur W. Schultz (Chairman), Harry N Abrams Inc, New York, pp. 96-107.
Horie, V., 1989, Materials used for glass conservation, in Conservation of Glass, Eds R. Newton and S. Davison, Butterworths, London, pp. 165-185.
Jackson, P.R., 1982, A dowelling technique for glass restoration, The Conservator, vol. 6, pp. 35-36.
Koob, S., 2006, Conservation and Care of Glass Objects, Archetype Publications, New York.
Newton, R., 1989, Deterioration of glass, in Conservation of Glass, Eds R. Newton and S. Davison, Butterworths, London, pp. 135-164.
Newton, C. and Logan, J., 1990 (revised 1997, 2007), Care of Ceramics and Glass - Canadian Conservation Institute (CCI) Notes 5/1, Canada.
Tennent, N. (Ed.), 1999, The Conservation of Glass and Ceramics: Research, Practice and Training, James & James (Science Publishers) Ltd, London.