Wet Materials
J. Carpenter, I. M. Godfrey, I. D. MacLeod and V. L. Richards
Introduction
Materials recovered from marine, freshwater or wet terrestrial sites pose quite different conservation problems to those excavated from dry sites. Both the extent and the type of deterioration that occur in wet environments differ significantly from those that take place under dry conditions.
Factors that affect the extent of deterioration in a wet environment include the following:
- temperature;
- degree of oxygenation;
- presence of bacteria, fungi and other organisms;
- light;
- period of immersion or burial;
- nature of the material surroundings;
- extent of burial/exposure cycles; and
- development of encrusting concretions.
Warm, moist conditions usually increase degradation processes whereas cool, dry conditions are less damaging. Damp surroundings encourage breakdown of materials through chemical and biological processes. Water is essential for many chemical reactions, the corrosion of iron and the hydrolytic breakdown of the cellulose fibres of wood and textiles being examples of two such reactions. Moisture also encourages fungal and bacterial attack on susceptible materials.
The aqueous environment from which an object has been recovered will give an indication of the type and extent of degradation to which an immersed object has been subjected. Preservation chances improve when the temperature is low and when there are low levels of dissolved oxygen and light. Wood buried in low oxygen surroundings for instance, has been known to survive in marine environments for thousands of years. On the other hand, timbers exposed on the seabed will be broken down rapidly by marine borers, microorganisms, chemical attack and the abrasive action of waterborne particles.
The nature of the material surroundings may affect deterioration significantly. The chemical composition of the burial environment (e.g. acidity, salt content, dissolved oxygen levels), the presence of a sacrificial metal in contact with a less reactive metal (leading to protection of the latter) and the diffusion of metal corrosion products into porous organic objects that then either stain the objects or in some cases protect the objects from biological deterioration are just some of the possible effects of differing burial environments.
For objects found in wet terrestrial sites additional factors such as soil acidity and the chemical characteristics of the ground water influence degradation processes.
Some objects are fortunate enough to be afforded protection from their surroundings by being buried, encapsulated in concretion (a cement-like corrosion product) or by being under the protective influence of another material that is present in a neighbouring object. The process of removing objects from these protective surroundings upsets these relatively stable states. Unless appropriate conservation steps are taken immediately accelerated degradation will result.
Although the emphasis in this section will be on materials recovered from marine sites, the same principles can be applied to objects recovered from freshwater and wet terrestrial sites. Changes are expected in the condition of materials from these different environments with respect to the nature and extent of degradation and obviously the degree of salt contamination.
As wet ceramics and glass have been discussed in separate chapters dealing with those particular material types, this chapter will focus on organic materials and metals. Materials that contain at least an organic component include wood, leather, bone, ivory, horn, textiles and any materials made from natural fibres such as rope and matting. Organic materials may be found as discrete objects or as parts of composite objects that also incorporate other materials such as metals or ceramics.
Identification
It is essential to identify the materials from which an artefact is made. This information, when combined with knowledge of the environmental conditions to which an artefact has been subjected, will assist in determining its likely condition before it is handled and treated. Once identified, specific recommendations can be made regarding storage and treatment procedures.
Often familiarity with the type of object is a reliable initial indicator of its usual material composition or likely material of manufacture. The types of corrosion products associated with metallic objects are also good indicators. The colours and formation of corrosion products can provide information about the material type and the burial conditions to which an object has been subjected. In aerobic conditions for example, iron corrodes to produce characteristic orange-brown rust colours whereas copper corrosion under similar conditions produces distinctive blue-green corrosion products. If the site is anaerobic however, metals are generally blackened as metal sulphide corrosion products predominate.
Following excavation and recovery artefacts may visibly react to the changes in environmental conditions and in doing so may provide information about the artefact’s composition. Silver corrosion products for example may blacken when exposed to light. Corrosion products also sometimes produce smells that assist in materials identification. Iron corrosion products for example, have a characteristic odour.
Note that a magnet can be used to detect the presence of residual iron or magnetite.
On-site Storage Considerations
Following excavation, an artefact is exposed to a set of conditions quite different from those of its former burial environment. It will be vulnerable to both physical damage and chemical changes. For example a corroded iron object recovered from a reducing, anaerobic environment will usually exhibit black or grey corrosion products. Subsequent exposure to aerobic conditions results in a transformation to orange-brown rust. With time, the expanding products of the oxidation process are capable of destroying these objects.
It is important to provide stabilising conditions as soon as possible following excavation and to be prompt when recording information about recovered objects lest changes occur in the meantime. If the documentation procedure is prolonged then keep artefacts wet with a fine water spray and/or dampened cloth covers.
As the extent of material degradation of recovered artefacts is not always immediately apparent, handle objects with great care to minimise further damage. Label all artefacts and storage containers and include precautionary comments if required.
As there are too many possible scenarios for excavated wet objects, only basic guidelines can be given.
Metals
The guidelines listed below will afford recovered wet metal objects some protection:
- store in water, with freshwater preferred to salt water. Do not use soft water that is low in mineral salts as it will promote corrosion of lead and lead alloys;
- only store like metals in the same container;
- retain adherent concretions;
- remove the majority of marine organisms and plant matter if possible;
- use common sense if putting more than one object in a container with smaller, lighter objects on top;
- store fragile objects separate from more robust objects; and
- do not store metals in the same container as organic materials unless they are part of an inseparable, composite object.
Further details are provided for individual metal types later in this chapter.
Organic Materials
Adhere to the following guidelines for organic materials:
- keep the object wet;
- support large or fragile objects;
- wrap fibrous objects with smooth materials that are unlikely to mark or otherwise damage the surface;
- retain concretions;
- separate objects that are contaminated with iron corrosion or similarly coloured products from uncontaminated objects;
- do not expose objects to direct sunlight;
- store objects at low temperatures (4 - 5 °C) and in the dark where possible;
- change the storage water if biological growth is observed;
- use fungicides in solutions if storage is prolonged and biological growth is a problem. Do not add any fungicides to a storage solution if the object is to be used for subsequent analyses such as radiocarbon dating; and
- begin treatment as soon as possible after recovery.
Upon recovery, do not allow organic materials to dry out. In most cases irreversible damage will be done if this occurs. Wood for example, is prone to shrinking, cracking and warping upon drying (Figure 1). Many objects recovered from buried wet sites appear black and it is sometimes difficult to identify the object’s composition. If unsure of the nature of the object, keep it wet.
Figure 1: (a) Conserved wood fragment from the wreck of the Rapid (1811) after drying.
(b) Untreated Rapid wood after drying. The red outline in both images shows the shape of each piece before drying.
When lifting large objects such as wood, or fragile materials such as leather and rope:
- support them on planks, netting or similar materials. Place padding between large objects and the support. If object surfaces are very soft use support materials that will not leave an impression in the surface. Netting without padding may leave damaging marks;
- lift objects by gripping the supports, not the objects; and
- protect against accidental damage by cushioning any fastenings or retainers used to keep the object in place. Provide padding and additional support for any protuberances.
To prevent fibre loss and damage due to water movement when storing fibrous materials such as rope and textiles:
- wrap them in materials such as tulle which will not snag the fibres;
- store them in lidded containers completely filled with water to minimise damaging ‘slopping’ movements; and
- alternatively wrap the objects in tulle and then wrap them again in water-saturated paper towel or cloth and then place the wrapped object in a sealed polyethylene bag or container.
Horticultural gel media products used to retain moisture around cultivated plants are also useful for wet organic materials storage. A wrapping is still advised if the gel is likely to intermingle with fibrous or other similar materials.
Store small objects in polythene bags or containers. Initially water from which the object was recovered may be used to keep the object wet. Placing one bag inside another provides additional protection against both drying out and physical damage.
Use freshwater for longer term storage, in conjunction with an appropriate fungicide if biological growth cannot be eliminated by changing solutions. A range of fungicides is available including Kathon (used as a 0.1 – 2 % solution), Panacide (0.2 %), Shirlan Plus (1 %), Dowicide (1 %), Densil P (0.3 %) and thymol crystals (excess in tap water). The choice of fungicide depends on the nature of the material in storage. Dowicide and Panacide for instance, are not suitable for use with textiles or leather due to their alkaline character (Pearson 1987). Exercise care when handling fungicides as many are toxic. Use appropriate personal protective equipment and observe all precautions described in their respective material safety data sheets.
Begin treating wet materials as soon as possible after their recovery. Long-term storage is not recommended as deterioration not only continues but often accelerates during such storage. If long-term storage is unavoidable, attempt to maintain conditions which minimise deterioration. Keep temperature, light and oxygen levels as low as possible. The latter two factors may be assisted by the use of narrow, deep storage tanks that conform as closely as possible to the dimensions of the object. Use refrigeration to minimise biological degradation and to reduce the need for biocides but do not freeze objects. Do not expose objects to direct sunlight (Keene 1977).
Where possible, retain adherent concretions until treatment begins.
If polythene bags, tanks and other containers are not available, wrap materials in dampened, water-absorbent material such as cotton cloth or hessian (or Burlap in the USA), wrap them again in plastic and seal joins with adhesive tape. Self-sealing plastic cling wrap is also good for maintaining moisture. Inspect the wrapped materials regularly to check that the object is still moist and that there have been no outbreaks of mould.
Packing and Transportation
As for on-site storage, care and common sense are needed when packing wet materials for transportation to a treatment laboratory. Find a balance between the maintenance of an appropriate storage environment, support for the object and the weight of the object and its storage medium. If 100 % relative humidity can be assured, actual immersion in water may not be necessary thereby reducing the overall weight. Avoid using biocides in solutions used to transport objects as the chemicals may have detrimental consequences should they leak. Pack objects according to the type of conditions that they are likely to encounter while in transit so that they will not be damaged. Packing to prevent damage to objects being transported by sea for example, is challenging if damage is to be prevented in rough sea conditions. An overview of packing and transportation is provided by Leskard (1987).
Containers used for wet packing must be waterproof with lids that form tight seals. Plastic containers, liners and bags, although still susceptible to physical damage by puncturing or fracturing are preferred to glass containers for transportation.
Avoid problems of excess weight or the possibility of damage being caused to an object by water movement by using damp wrapping and padding materials.
Do not use packing materials that will contribute to the further deterioration of the object or that are difficult to remove when the object is unpacked. Improvise if suitable padding materials such as bubble wrap are not available. Use materials such as the original supporting matrix (e.g. soil, concretion), sawdust, seaweed, undyed fabrics and even moss to maintain a damp supportive environment (Lucas 1982). Preferably wrap the object prior to padding especially if the improvised packing/padding materials could deteriorate and contaminate the object during prolonged transit.
When using improvised packing/padding materials be aware that most countries will not allow the importation of foreign soils and plant materials. Quarantine delays may compromise the preservation of artefact materials.
Do not expose objects to extreme conditions such as direct sunlight during transport. Use insulating materials around supportive packaging to avoid this problem.
Metals
Wet metals are generally much more reactive than their dry equivalent. Of the factors that affect the deterioration rate of wet materials three are probably more significant for metals than they are for other materials. These are the:
- development or otherwise of a layer of encrusting material;
- concentration of dissolved oxygen available to the metal surface; and
- location and relationship to other materials.
Some metal compositions encourage biological growth. Iron objects provide a good substrate for marine encrustation and often become encased in cement-like concretions. By providing a barrier to the inward diffusion of dissolved oxygen and salts, concretions serve to slow destructive corrosion processes (Figure 2). They also provide reinforcement for the weakened structure of encapsulated objects and provide protection against physical damage that may be caused by moving sand and wave action. Copper and its alloys tend to be toxic to marine organisms and encrustation is generally much less on objects made from these materials than on iron and its alloys.
Figure 2: Examples of bronze astrolabes. One was protected by concretion while the other was exposed to changing conditions on the sea bed.
Dissolved oxygen concentration is influenced by factors such as salinity, temperature and depth of burial in the sediments. The depth of the water at an archaeological site becomes significant as it will affect the ambient environmental conditions. As water movement and turbulence are greater in shallower sites more oxygen is supplied to the metal surfaces leading to increased corrosion rates.
The least reactive of two metals in contact or close proximity can be protected by the other. In this process the most reactive metal or alloy corrodes while protecting the least reactive metal at the same time. For example if copper and iron were in contact the iron would preferentially corrode and the copper would be protected.
A beneficial example of this process is demonstrated by the use of sacrificial anodes attached to the hulls of ships. Similar protection can exist with the metal of an artefact. Unfortunately it will frequently mean that another part of the artefact, or a connected metal artefact, will suffer more severe corrosion to provide this protection. Note that a sacrificial anode in electrical connection with an iron object that remains submerged in seawater will protect the iron object from further corrosion and also begin its desalination in–situ.
Metals and their alloys most commonly recovered from wet sites include gold, silver, copper, lead, tin, pewter, iron and aluminium. Of these pure gold is the most stable and aluminium the most reactive (see Appendix 8). The following sections contain information on the identification, handling and stabilisation of these more commonly recovered metals. The current recommendation concerning the initial storage of wet metals is to maintain them in a wet state.
Gold
Identification
The appearance of gold and its extensive use in jewellery manufacture usually allow this material to be easily identified. It is possible however, that corrosion products from any alloyed metals, commonly copper and silver, will disguise the gold appearance. Alternatively selective corrosion of these metals can cause surface enrichment of the gold component giving an impression that the artefact is very pure.
Handling
As pure gold is soft, mishandling can damage the surface and/or distort gold artefacts. Alloyed copper and silver impart strength to uncorroded gold artefacts but selective corrosion of these metals causes structural weakening.
Initial Stabilisation
- store in freshwater as most gold artefacts are alloys. Pure or uncorroded gold may be rinsed with fresh water and stored dry;
- store any gold which shows signs of copper corrosion either in freshwater or preferably in an aqueous benzotriazole solution (BTA, 10 g in 1 L of water); and
- store any gold which shows signs of silver corrosion in freshwater so that any potentially recoverable surface details contained within the corrosion products will be retained.
Silver
Identification
Silver corrosion products are usually grey or black. Silver salts react in the presence of light and in some instances blackening of freshly exposed silver corrosion products has been observed in strong light. Blue-green copper corrosion products may also be present on alloyed silver artefacts recovered from aerobic sites.
Handling
Silver artefacts from freshwater sites are more likely to retain structural strength. As more extensive corrosion is expected in artefacts recovered from saltwater sites, handle these materials carefully.
Retaining corrosion product layers is very important as archaeological information can be preserved and recovered from them.
Initial Stabilisation
To stabilise silver recovered from a wet site, observe the following guidelines:
- store objects in freshwater initially. If the silver is sound, clean and uncorroded then rinse the artefact with freshwater and allow it to dry;
- keep the silver in freshwater if the artefact is mineralised or possesses corrosion product layers; and
- store silver-copper alloys in a solution of benzotriazole (10 g in 1 L of water).
Copper and its Alloys
Identification
In aerobic and particularly alkaline (high levels of carbonate) conditions, the presence of copper is often indicated by green-blue corrosion products (copper hydroxycarbonates) which frequently stain the surrounding burial material (Figure 3). Under anaerobic conditions protective layers of black copper sulphide are formed instead.
Figure 3: Before and after treatment of the bell from the Cambridgeshire (1875).
Due to the toxicity of copper compounds towards marine organisms copper objects usually possess little encrustation or concretion.
Selective corrosion in copper alloys can result in the formation of a surface corrosion layer derived from the alloying metal. This complicates identification. For example, under low oxygen conditions the galvanic corrosion of bronze produces a grey-coloured, tin-rich corrosion layer. Weight loss, diminished strength and an increase in porosity result. Corroded bronze objects can appear to be made solely of copper if there is extensive destannification. Dezincification of brass has a similar result.
Handling
Although artefacts made of copper and its alloys are frequently recovered in very good condition, support will be needed if extensive corrosion of sheet metal has occurred or if thicker more robust objects have been severely eroded. Handle cast brass and bronze objects with caution as sometimes they will have soft and weakened surfaces due to selective corrosion of the more reactive component metals of the alloys.
Initial Stabilisation
To stabilise copper artefacts, follow the procedures outlined below:
- store recently recovered and structurally sound copper objects in freshwater;
- immerse badly corroded and structurally unsound copper objects in a solution of sodium sesquicarbonate (10 g of sodium carbonate and 10 g of sodium bicarbonate in 1 L of freshwater). This washes out the chlorides and stabilises the object.
Iron and its Alloys
Identification
The following features indicate the presence of iron:
- a large concreted mass or concretion layer (cement-like corrosion product combined with burial material); and
- orange-brown rust colours (in aerobic conditions).
Iron found in a wet site is usually weakened, with the loss of strength related directly to the extent of corrosion. It is important to distinguish between cast and wrought iron as the effect of corrosion processes is different.
Carbon, some in the form of graphite, is a constituent of cast iron. This inert substance does not corrode and often will help retain the original physical shape and dimensions of the object even when the iron content has decreased significantly. Corrosion is directly associated with weight loss and diminished structural strength. Concretion protects structurally weakened surfaces and reinforces corroded cast iron artefacts.
The corrosion pattern of wrought iron is determined by its manufacturing process. Since the metal is forged, beaten and folded into shape, the object acquires inclusions of impurities and different qualities of metal are laid down. In addition, manufacture sometimes imparts stresses to the metal which become a focus for corrosion activity. Consequently corrosion processes produce a furrowed surface not unlike the weathered grain of wood. The amount of uncorroded residual metal and the extent of interconnection of the remaining structure usually determines how well an object survives.
Handling
Handle all concretions carefully as the encapsulating medium, while providing protection for the encased iron object(s), may also contain other fragile components or materials. Provide support and do not lift concretions by protuberances.
Initial Stabilisation
To stabilise iron objects recovered from wet sites:
- store them initially in either the water from which they were excavated or in freshwater which contains an alkaline agent such as sodium carbonate (washing soda);
- for long-term storage, keep iron in a solution of sodium hydroxide (caustic soda, 20 g in 1 L of water) or sodium carbonate (50 g in 1 L of water);
- store iron from freshwater sites initially in distilled or deionised water; and
- in the short term, place small iron objects in an airtight container with a desiccant such as self-indicating silica gel.
Note that on safety grounds it is generally advisable to transport iron artefacts in water and not in caustic or other alkaline solutions.
Alkaline solutions will attack composite iron objects such as tinned wares, enamelled wares and any associated organic materials and should not be used to store composite objects which contain these components.
Lead
Identification
Indicators that an object may be made of lead include:
- a high weight-to-size ratio;
- grey appearance;
- cream-white encrustation (aerobic conditions); and
- areas of black corrosion products (anaerobic conditions).
In wet alkaline environments a combined layer of lead sulphate and calcium carbonate may form. This protective cream-white layer may have surrounding debris attached.
Under anaerobic conditions an inert layer of grey-black lead sulphide will form. Aerobic conditions can transform this layer to lead sulphate. Passivation of lead in aerobic and anaerobic conditions protects it from further rapid corrosion.
Lead is susceptible to attack by organic acids however, such as those found in peat and clay deposits and those formed when organic matter decays.
Handling
Handle lead artefacts with gloves as lead and its corrosion products are toxic. Wear a dust mask to avoid inhalation of dry corrosion products.
The passivating nature of lead corrosion products usually ensures that these objects are often found intact. The innate softness of lead means that it is highly susceptible to physical damage. Lead objects are often found deformed, torn, or abraded.
Handle lead objects carefully to prevent further damage to the surface of the lead or to its shape. Large objects, especially those made of sheet lead, will need support to prevent bending under their own weight. Corrosion products impart little reinforcement and easily fracture and detach if bending occurs.
Initial Stabilisation
To stabilise lead objects:
- store in seawater or freshwater containing sodium sulphate (1 g in 1 L of water).
- remove any marine growth to prevent attack from organic acids produced during the decay of these organisms; and
- after treatment and when dry, store away from potential sources of organic acid vapours such as acetate adhesives and unsealed wood including composite wood products such as chipboard.
Do not place lead in distilled, deionised or soft tap water as it will speed up corrosion. If untreated lead is stored dry it may make later removal of concretion more difficult.
Pewter
Identification
Depending on environmental conditions, pewter recovered from the sea can be found in excellent condition. Pewter surfaces are usually grey to dark grey in colour. Cracks and damaged surfaces will sometimes be defined by contrasting lighter corrosion products. Surfaces may exhibit a uniform-sized, blistered appearance with occasional large, sporadically distributed blisters.
Handling
As pewter is a relatively soft alloy it is susceptible to physical damage. Handle it carefully. Do not force distorted pewter artefacts back into shape as the metal may have become brittle.
Initial Stabilisation
To stabilise pewter artefacts recovered from wet sites:
- keep wet and remove any marine growth to avoid potential corrosion problems associated with their decay; and
- store in freshwater containing sodium sulphate (1 g in 1 L of water) and BTA (10 g in 1 L of water).
As for lead, avoid storing pewter in deionised water and soft tap water as it increases corrosion rates.
Tin
Identification
The type of object (e.g. an ingot or a food can) can indicate the use of tin in its manufacture or construction. Aerobic tin corrosion products are usually crumbly and whitish in colour.
Handling
Handle carefully objects made from either tin or coated with tin plate so that the corroded surfaces are not damaged and the intact metal is not subject to additional stresses. Support thin objects.
Initial Stabilisation
Initially store tin artefacts in the water from which they were recovered.
Aluminium
Identification
Aluminium has a light grey or silvery grey, naturally oxidised surface.
Handling
Aluminium objects need support when perforated by corrosion. Avoid exposing aluminium to alkaline and acidic substances and solutions.
Initial Stabilisation
Store all aluminium and aluminium alloys in the water from which they were recovered.
Treatments
The treatment of wet metals is a specialised area and one in which research is ongoing, particularly in the area of composite objects. Consult a conservator and/or conservation websites before attempting any treatment of wet metals to obtain the most up-to-date advice on particular techniques and treatments.
Storage and Display
For preservation it is important that artefacts are kept in a suitable environment following treatment. The optimum is not always possible as museum preferred standards are difficult to achieve. An awareness of the best environmental conditions for an artefact will however, allow collectors to assess their own particular environments and strive for the best possible conditions. It is generally easier to establish a suitable environment when artefacts are placed in storage than when they are on display.
Principles for storing and displaying stabilised metals are described elsewhere (see the chapter Metals). Specific details pertaining to particular metals may be found in the relevant sections of that chapter. In some cases formerly wet metals that have been stabilised will be more susceptible to damage by the environment and by inappropriate materials used in storage and display areas than will their historic counterparts.
Organic Materials
Wood
Identification
In some cases, as in the recovery of a blackened object from an anaerobic site, it is not always easy to identify the material type from which the object was made. The appearance of an object and its probable usual use, along with the fibrous nature of wood usually allow wooden objects to be identified.
Consult timber specialists if it is important to identify the wood species from which an object has been made. By examining either a polished end-grain surface or by preparing a microscope slide of the end-grain, the identity of a wood sample can usually be determined. Take samples from the least obtrusive part of the object to minimise damage to the object. Identification is relatively straight forward if the sample’s cell structure is in good condition and the wood is not heavily stained or blackened.
Handling
Ascertain the condition of wet wood before handling it as its appearance can be deceptive. The condition of recovered wet wood can vary from solid to mushy. Handle it accordingly, especially as the surface is usually soft. Use a very thin needle to determine the ‘softness’ of the surface and interior of the object. Test the softness in a number of places so that the overall condition of the wood can be determined. Being bulked by water, a wooden object can appear quite robust when in reality it is highly degraded and in need of support before it is moved.
Follow the general guidelines described in the Introduction to this chapter (On-site storage considerations) for wooden artefacts.
Initial Stabilisation
To stabilise wood recovered from wet sites:
- keep objects wet;
- maintain the support provided by water for smaller fragile wooden objects recovered from underwater sites and raise them in a suitable container, preferably sealed with a lid;
- store objects at low temperatures and in the dark if possible;
- change the water if biological growth is observed;
- use freshwater with a fungicide if biological growths are persistent and storage is prolonged (sample wood before adding biocides if a subsequent dating determination is planned); and
- store metal-contaminated wooden objects separately from non-contaminated objects.
Wet wood must be kept wet once recovered. Irreversible damage will occur if it is allowed to dry before treatment (see Figure 1). Use baths, plastic bags or containers, tubs or even wet cloths covered with plastic to keep artefacts wet.
Initially store wooden objects in water from their recovery sites. If sufficient freshwater is available then use it for objects excavated from a marine environment so that desalination can begin. Incorporate a fungicide such as Kathon (0.1 – 2 %) in the solution if biological growth cannot be controlled by changing water, refrigeration or other means of low temperature storage.
Leather
Identification
The ease of identifying leather is directly related to its condition. Highly degraded wet leather may appear as nothing more than mush or just as a mass of fibres held together by corrosion products. Often however, wet leather retains its shape and its characteristic appearance, making its identification relatively easy.
If an object is thought to be leather but there is some doubt, confirm its composition by infra-red spectroscopic analysis. This is a specialised technique which only needs a pin-head sized sample for the analysis.
Handling
When handling leather objects:
- use supports; and
- wrap in gauze or similar material and sew the edges together.
Handle thin or fragile leather extremely carefully as it is prone to tearing while wet. The best method for handling is to insert a support beneath the leather in its storage solution and to ‘float’ the leather onto the flat surface. Use the support, not the leather, when lifting the object.
Wrap small leather objects in soft gauze (e.g. tulle) and sew the edges together to make handling easier and to ensure that all parts of the object will be retained.
Initial Stabilisation
To stabilise leather from a wet site:
- store wet;
- store at low temperatures and in the dark if possible;
- change the water if biological growth is observed;
- store in freshwater containing an appropriate fungicide if biological growths persist; and
- store metal-contaminated leather objects separately from uncontaminated objects;
Do not allow wet leather to dry out before it is treated. For marine objects, seawater is acceptable for short-term storage before transferring them to a freshwater solution. If a fungicide is needed, only use pH neutral or slightly acidic fungicides such as Densil ‘P’ as alkaline fungicides contribute to leather breakdown. If possible store wet leather in a refrigerator or similarly cool environment. Do not store wet leather for long periods as deterioration processes continue.
Bone, Ivory and Horn
Identification
Identifying bone and related materials is often complicated by their dark colouration (due to burial in predominantly anaerobic conditions) or staining by corrosion products. As a consequence, these materials have been mistaken for materials such as wood and ceramics. If bone and ivory objects are incorrectly identified, they may be treated inappropriately. Use microscopic examination to distinguish these materials (Penniman 1952).
If microscopic identification is inconclusive, use infra-red (IR) spectroscopic analysis. IR analysis will allow bone and ivory to be clearly distinguished from other material types. The IR spectra of bone and ivory are similar however, particularly if samples are degraded.
Handling
Bone, ivory and horn objects are often fragile and require careful handling.
If these objects have been closely associated with iron objects they are often heavily contaminated with iron corrosion products. These often create a hardened exterior surface which can hide a multitude of problems, including a highly degraded core (Figure 4). Take care not to dislodge these protective outer layers as they can be quite brittle. Wrap ivory and bone objects in bubble wrap or similar materials for protection.
Figure 4: (a) Tusk fragment from the Vergulde Draeck (1656) shipwreck showing contamination with iron corrosion products.
(b) Cross-section of the same fragment showing different areas of deterioration in the inner parts of the tusk.
Initial Stabilisation
To stabilise bone, ivory or horn materials recovered from wet sites:
- store wet;
- use water from the excavation environment or freshwater;
- store at low, but not freezing temperatures if possible; and
- store metal-contaminated objects separately from uncontaminated objects.
Ensure these objects remain wet as the laminated structures of ivory and horn are usually coherent while wet but tend to delaminate upon drying. Short-term storage in the water from which the objects were recovered is acceptable.
After initial storage, place the items in freshwater at low temperatures (about 5 °C) to begin desalination, thus avoiding the need to incorporate a fungicide. This is important as the complex nature of these material types (inorganic and organic components with different pH tolerances) means that the choice of an appropriate fungicide is very difficult.
Textiles
Textiles include materials made up of cellulosic fibres such as cotton, flax, hemp, jute and sisal, and proteinaceous fibres such as wool and silk. Fabrics, rope and matting have all been recovered from wet sites. Usually textile materials will survive in a wet environment only if the site is anaerobic or if they have been protected from their surroundings by encapsulating concretions.
Identification
Identifying fibres usually requires a high power, good quality microscope and access to appropriate references which highlight the features of different fibre types. As textile fibres from wet sites are usually highly degraded, choose the best fibres for examination to ensure that characteristic features are still evident.
Handling
When handling wet textiles:
- support them so they do not carry their own weight;
- use flat supports for flattened textiles; and
- use sewn netting for three-dimensional objects.
Of the organic materials wet textiles are often the most difficult to handle because of their fragility and inability to maintain their own shape when removed from their aqueous environment. At all stages of treatment most textiles need some sort of support to maintain their structural integrity.
Support flat textiles on a glass plate or similar, separated from the support by interleaving with synthetic papers or screening material.
Three-dimensional textiles like rope usually need to have a support sewn around them to maintain the overall integrity of the object and to prevent fibre separation and loss. Gauze screening, tulle for example, is preferred as it allows relatively easy access for cleaning and offers little resistance to both the outward diffusion of dirt, salts and corrosion products and to the inward movement of consolidants and other treatment chemicals.
Initial Stabilisation
To stabilise wet textiles:
- store wet, using either the water from which the textile was recovered or freshwater;
- support and wrap fibrous textiles while in the wet state;
- store at low temperatures and in the dark if possible; and
- consult a conservator for advice on incorporating appropriate biocides if longer term storage is likely.
Treatments
As many of the current treatments for wet organic objects require access to specialised equipment (analytical and technical) and research into treatments is on-going, an overview of some treatment processes are described rather than specific treatments for particular object types. Contact a conservator to obtain the most up-to-date information.
Generally, treatment of wet organic materials involves four distinct stages:
- cleaning - physical and chemical;
- desalination;
- consolidation; and
- drying.
Physical Cleaning
Clean the surfaces of wet organic objects before chemically treating them. This allows the material type to be determined, improves the rate of outward diffusion of any absorbed salts or corrosion products and improves both the object’s appearance and its permeability to stabilising consolidants.
During cleaning take care not to damage an artefact’s surface as it is important not to remove or disfigure potentially useful archaeological information.
Depending on the nature and condition of the object in question, either remove soil and similar materials by careful brushing in water or use ultrasonic agitation if appropriate. Monitor the removal of detritus through a microscope to ensure that surfaces of small valuable items are not damaged during cleaning.
To minimise the damage to the surface of soft materials like wood, bone and leather, use wooden and plastic scrapers rather than metal ones to remove more stubborn encrustations from the surface. Remove adherent concretion with dental tools or by gentle percussion. Wash off or carefully wipe debris from the surface while keeping the object wet at all times.
The fragility of wet textiles, including rope and matting, complicates their cleaning. Support these materials on either glass, perspex sheets or netting while cleaning them. As brushing will inevitably lead to fibre damage, use a fine spray of water or careful immersion (still on a support) to clean the object. Carefully applied ultrasonic techniques have also successfully cleaned these fragile materials. If possible conduct a test on a sample section before using ultrasonic techniques on a larger scale.
Chemical Cleaning
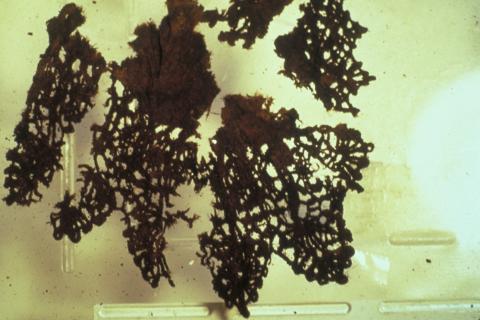
Organic objects excavated from wet sites will have absorbed substantial amounts of salts from their surroundings. For shipwreck objects the most commonly incorporated materials, apart from the ubiquitous salt, are metal corrosion products which originate from nearby corroding copper and iron artefacts (Figure 5).
Figure 5: (a) Lace from the Batavia (1629) shipwreck before treatment.
(b) The same sample after treatment to remove corrosion products.
In many cases incorporated iron corrosion products are not only disfiguring but chemically damaging to objects. In conditions of elevated relative humidity the presence of iron corrosion products and reduced sulphur in conserved wood for example, leads to the development of damaging acidic regions (Sandström et al 2003). Cellulose breakdown follows. Other cellulosic materials, such as rope and many textiles, are also at risk in the presence of iron corrosion products.
Treatments are aimed at removing these incorporated minerals from organic objects. This is achieved by using chelating or complexing agents to remove the intrusive corrosion products. The chemistry associated with these processes is not simple. It is essential to utilise the services of a conservator who can monitor treatments regularly to determine treatment effectiveness and the rate at which corrosion products are removed.
Desalination
Desalinate wet organic objects before any impregnation or consolidation treatment. Apply the procedures outlined earlier in this book (see the chapter Ceramics). Note that the more porous nature of organic materials means that desalination times will be less than for metals and glass. If necessary incorporate a fungicide in the desalination solution to protect objects while salts are being removed.
Consolidation Treatments
As organic objects deteriorate in a wet environment the soluble degradation products that are generated during this deterioration leach into the surroundings. In many cases the object is bulked by water which takes the place of the leached chemical constituents. This helps to maintain the shape of the object, but only while it is in its wet state. Unfortunately, as drying takes place supporting structures are lost and cell collapse, shrinkage and warping result.
The aim of consolidation treatments is to prevent this damage by progressively replacing the water in degraded organic objects with chemicals (consolidants and bulking agents) which will bond with the residual chemical constituents of the object. These consolidants provide objects with added structural integrity when they are dried.
If an organic artefact needs to be analysed, sample it before any chemicals such as fungicides or consolidating agents are used to treat it.
Drying
Damage to wet objects becomes obvious if drying is uncontrolled. Wood may warp, crack or shrink, ivory delaminate and leather shrink. Drying damage is irreversible for objects like wood, bone and ivory. Although leather objects can be retreated if their shrinkage is unacceptable (> 5 %), avoid this if possible.
The key to success in drying is to ensure that drying stresses are minimised. Vacuum freeze-drying is the best method to dry objects such as wood, rope and textiles as it gives a much better end product than air drying. This is because the freeze-drying process allows moisture to be removed as vapour directly from ice (sublimation). Drying stresses induced by the strong surface tension forces of water are avoided when the liquid state is bypassed in this way and any water–based consolidants cannot drain away as could happen if the ice reverted to liquid form.
Although vacuum freeze-drying is the most efficient technique, it requires the use of sophisticated and expensive equipment. It is used primarily for drying small objects. A frost-free freezer is also very effective at slowly freeze drying conserved organic objects without the need for an applied vacuum. Monitor the weight of objects as they dry to determine when the drying process is complete.
Controlled air drying is usually needed for larger objects. Controlled dehumidification over a prolonged period is the next best alternative to freeze-drying. This process may involve the use of expensive, electronically controlled dehumidification chambers or improvised materials to construct a chamber in which the temperature and relative humidity can be regulated. One simple way of slowing the drying process is to place objects under polyethylene sheeting, venting the saturated atmosphere that builds up under the plastic once a week. Alternatively, place small objects in plastic bags that have been pierced with pin-sized holes.
As mould formation is enhanced when the relative humidity is high, monitor the progress of drying operations, especially if objects are placed in or under plastic.
Preventive Conservation
Wet organic objects are often incomplete and are generally more degraded than their dry historic counterparts. Extra care is needed when handling, storing and displaying these objects.
Environment
Store and display treated wet organic artefacts under the conditions recommended for the material type in question. These may be obtained by referring to the relevant chapters of this book.
As for all materials, the key to improving the stability of wet organic objects is to maintain appropriate conditions that are as steady as possible. This is particularly important for wet organic materials, as in many cases the proteinaceous or cellulosic components of these materials will be substantially degraded.
Extra consideration and care should be taken with:
- cellulosic-containing objects that still contain iron corrosion products and reduced sulphur compounds; and
- objects for which incorporation of consolidating chemicals was necessary.
In both of these cases special attention may have to be paid to the environmental conditions to prevent continued deterioration of the treated artefact. For instance, under conditions of high relative humidity, oxidation and hydrolysis of incorporated iron corrosion products and sulphur compounds can lead to increased acidity and subsequent damage to dried, formerly waterlogged wooden objects. Maintain relative humidity levels of less than 55 % in order to prevent this type of deterioration.
The incorporation of polyethylene glycol (PEG) into an artefact will affect its response to its environment as low molecular weight PEGs are hygroscopic. Under high relative humidity conditions, PEG-treated artefacts may take on a sticky, greasy appearance. Mould formation and bacterial attack also are enhanced by the presence of a food source such as PEG and by high relative humidity conditions.
Handling
Many formerly wet organic archaeological objects are more degraded than their historic counterparts and consequently require more careful handling. For fragile objects, the construction of supports that allow them to be handled without actually touching the objects themselves is recommended.
Whatever the final choice for a support it should allow the object to be manipulated with minimal risk of damage. A support that is suitable for both storage and display is probably the ideal. Choose the support on the basis of the fragility of the artefact and its future use, be it storage, display or research (see the chapter Handling, Packing and Storage).
In all cases the media used to construct supports must be of archival quality and compatible with the artefact. Acid-free materials in contact with proteinaceous artefacts such as leather and silk, for example, should not contain alkaline buffering agents as these substances increase protein hydrolysis (see the chapter Preventive Conservation: Agents of Decay).
Summary
Metals
- Keep wet.
- Retain adherent concretions.
- Store separately from organic material.
- Do not store dissimilar metals in the same storage container.
- Consult a conservator for treatment advice.
Organics
- Keep wet.
- Retain concretions until treatment commences.
- Store objects contaminated with metal corrosion products separately from uncontaminated artefacts.
- Support fragile material on planks or netting.
- Lift by the supports, not by the object.
- Place cushioning between objects and the support material.
- Begin treatment as soon as possible after recovery. Seek advice from a conservator.
Bibliography
Godfrey, I., 2014, The Conservation of Waterlogged Wood - An Overview of Developments, in Shipwrecks around the World: Revelations of the Past, Ed. S. Tripati, Delta Book World, New Delhi, pp. 624- 657.
Grattan, D.W. and Clarke, R.W., 1987, Conservation of waterlogged wood, in Conservation of Marine Archaeological Objects, Ed. C. Pearson, Butterworths, London, pp. 164-206.
Jenssen, V., 1987, Conservation of wet organic artefacts excluding wood, ibid, pp. 122-163.
Keene, S., 1977, An approach to the sampling and storage of waterlogged timbers from excavation, The Conservator, vol. 1, pp. 8-11.
Leskard, M., 1987, The packing and transportation of marine archaeological objects, in Conservation of Marine Archaeological Objects, Ed. C. Pearson, Butterworths, London, pp. 117-121.
Lucas, D.A., 1982, On-site packing and protection for wet and waterlogged wood, Proceedings of the ICOM Waterlogged Wood Working Group Conference, Eds D.W. Grattan and J.C. McCawley, Ottawa, pp. 51-55.
North, N. A., 1987, Conservation of metals, in Conservation of Marine Archaeological Objects, Ed. C. Pearson, Butterworths, London, pp. 207-252.
Pearson, C., 1987, On-site storage and conservation, ibid, pp. 105-116.
Penniman, T.K., (1952), Pictures of Ivory and Other Animal Teeth, Bone and Antler, Occasional Paper on Technology, Pitt Rivers Museum, University of Oxford.
Sandström, M., Fors, Y. and Persson, I., 2003, The Vasa’s new battle: Sulphur, acid and iron, Vasa Studies 19, The Swedish National Maritime Museums, Stockholm.